Organic–inorganic lead halide hybrids are promising materials for solid-state lighting, e.g., light-emitting diodes (LEDs), fluorescent sensors, or displays. However, bulk lead halide hybrids generally have a relatively low photoluminescence quantum efficiency (PLQE). Reducing these materials to one- or two-dimensional nanostructures (i.e., quantum dots or nanosheets) can improve their efficiency. Doping them with other elements is another approach to optimize the material’s properties. However, both methods usually lead to unstable colloidal lead halide hybrid nanocrystals.
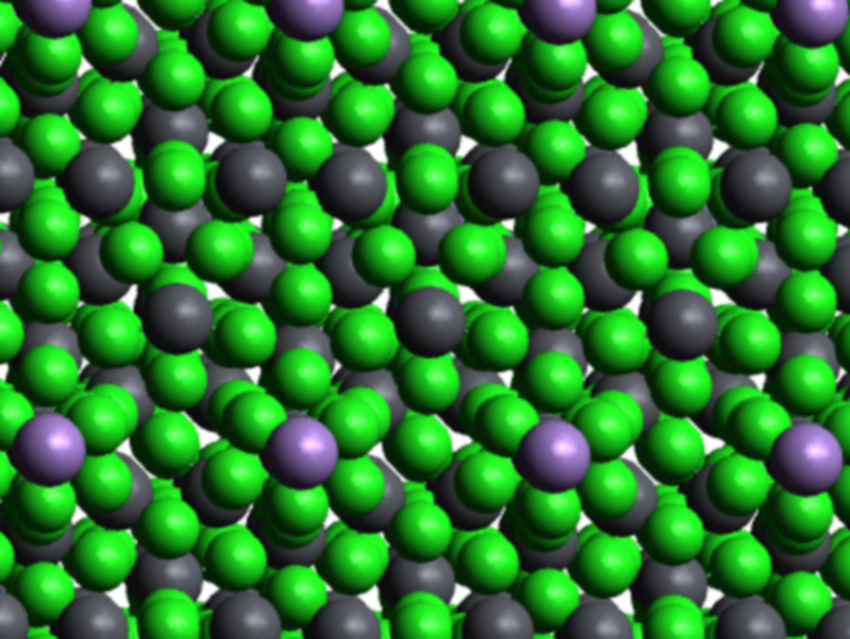
Junhua Luo, Lina Li, Chinese Academy of Sciences, Fujian, and colleagues have synthesized a two-dimensional Pb–Mn heterometallic halide hybrid, (C5H14N2)2Pb4MnCl14, by placing Mn2+ into the pure lead halide prototype (C5H14N2)2Pb5Cl14. The resulting Pb–Mn product features a 2D heterometallic halide layer configuration of the type [Pb4MnCl14]∞. These “heterometallic halide nets” are unique structures which facilitate efficient energy transfer. This results in a highly efficient red emission.
The PLQE of the Pb–Mn halide hybrid is up to 32 %. In contrast, that of the prototype pure lead compound is below 1 %. The structure of the heterometallic material maintains its stability and PLQE even at high temperatures and long exposures to air.
- Tailored Synthesis of an Unprecedented Pb–Mn Heterometallic Halide Hybrid with Enhanced Emission,
Yu Peng, Lina Li, Chengmin Ji, Zhenyue Wu, Sasa Wang, Xitao Liu, Yunpeng Yao, Junhua Luo,
J. Am. Chem. Soc. 2019, 141, 12197–12201.
https://doi.org/10.1021/jacs.9b04829