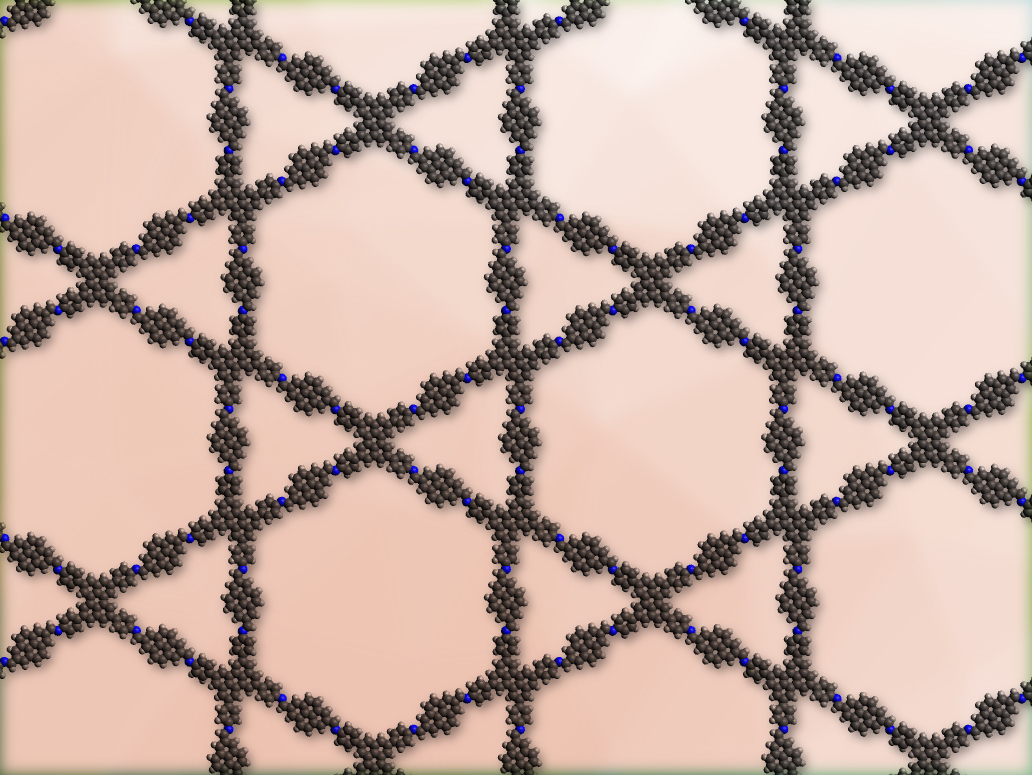
Covalent organic frameworks (COFs) are porous crystalline networks made from organic building blocks. They can, for example, be linked by imines. Usually, these imine groups do not have a function besides connecting the COF’s building blocks.
Florian Auras, University of Cambridge, UK, and colleagues have used the reactivity of imine linkers to create COFs that can be used as acid sensors. The team used perylene tetraaniline building blocks to synthesize COFs with imine bonds and a star-shaped internal pore structure (pictured). The COFs were synthesized using a solvothermal method for the co-condensation of 2,5,8,11-tetrakis(4-aminophenyl)perylene with different dicarbaldehydes (either phenylene-, naphthalene-, or pyrene-based).
In their free-base form, the COFs show red or yellow photoluminescence. If the imine bonds are protonated, the absorption of the materials is shifted further towards the red and near-infrared (NIR) parts of the spectrum and the luminescence is drastically reduced. The structure of the COFs is retained during protonation and deprotonation. Due to the resulting color change, thin films made from the COFs can be used as highly sensitive acid vapor sensors. The protonation can be reversed by exposing the COF to triethylamine vapor and washing it, which makes the sensors reusable.
- Perylene-Based Covalent Organic Frameworks for Acid Vapor Sensing,
Laura Ascherl, Emrys W. Evans, Jeffrey Gorman, Sarah Orsborne, Derya Bessinger, Thomas Bein, Richard H. Friend, Florian Auras,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b08079