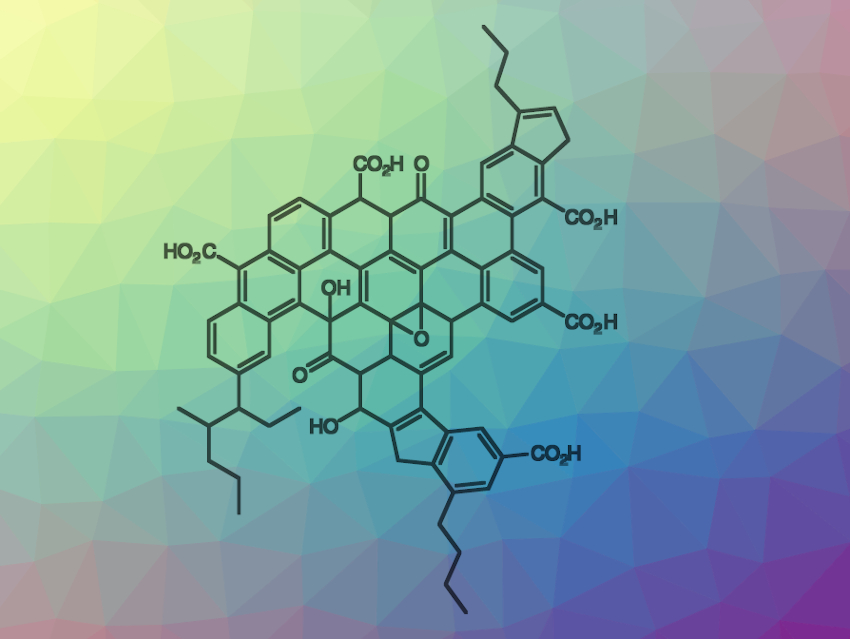
Asphaltenes, complex polycyclic aromatic hydrocarbons left in the distillation residue after mineral oil processing, are a cheap and abundant carbon-rich material. Carbon-rich oxidized graphene has been used as a catalyst for various reactions. However, this material lacks stability under high temperatures or harsh reaction conditions.
Hyosic Jung and Christopher W. Bielawski, Institute for Basic Sciences (IBS) and Ulsan National Institute of Science and Technology (UNIST), both Ulsan, Republic of Korea, have developed catalytic processes which use oxidized asphaltenes instead of oxidized graphene. Asphaltene oxide was synthesized according to Hummer’s method using potassium permanganate and sulfuric acid. A product containing around 13 wt% oxygen in various oxygenated functional groups (example pictured) was isolated.
The asphaltene oxides were tested as catalysts for the condensation of benzyl alcohol, a Claisen-Schmidt condensation, and the cross-coupling reaction between xanthene and veratrol. The products of all three reactions were obtained in good yields. Unsuccessful reactions using non-oxidized asphaltenes or organic acids as catalysts showed that the main catalytic activity is caused by the asphaltene oxide and not by trace-metal impurities in asphaltene or by simple acid catalysis.
Unlike graphene oxide, asphaltene oxide can also be used in microwave-assisted reactions. Under microwave conditions, the condensation of benzyl alcohol was faster and gave better yields than without microwave irradiation (90 % vs. 53 %). The microwave-based Fisher-indole synthesis between cyclohexanone and phenylhydrazine was also performed with good yields.
Productive uses for as many fractions of mineral oil as possible are desirable for economical as well as environmental reasons. The researchers showed that asphaltenes from heavy crude oil fractions can be transformed into catalysts by a simple oxidation reaction. These catalysts show catalytic activity similar to graphene oxides, while providing superior stability.
- Asphaltene oxide promotes a broad range of synthetic transformations,
Hyosic Jung, Christopher W. Bielawski,
Commun. Chem. 2019.
https://doi.org/10.1038/s42004-019-0214-4