Compounds with trifluoromethyl groups have useful properties, e.g., for pharmaceutical and agrochemical products. These groups can be introduced into molecules, e.g., by trapping ·CF3 radicals with alkenes, combined with a second functionalization to give 1,2-difunctionalized products. There are enantioselective variants of this type of reaction, as well as analogous enantioselective 1,6-difunctionalization reactions. However, enantioselective 1,5-difunctionalization reactions are more challenging.
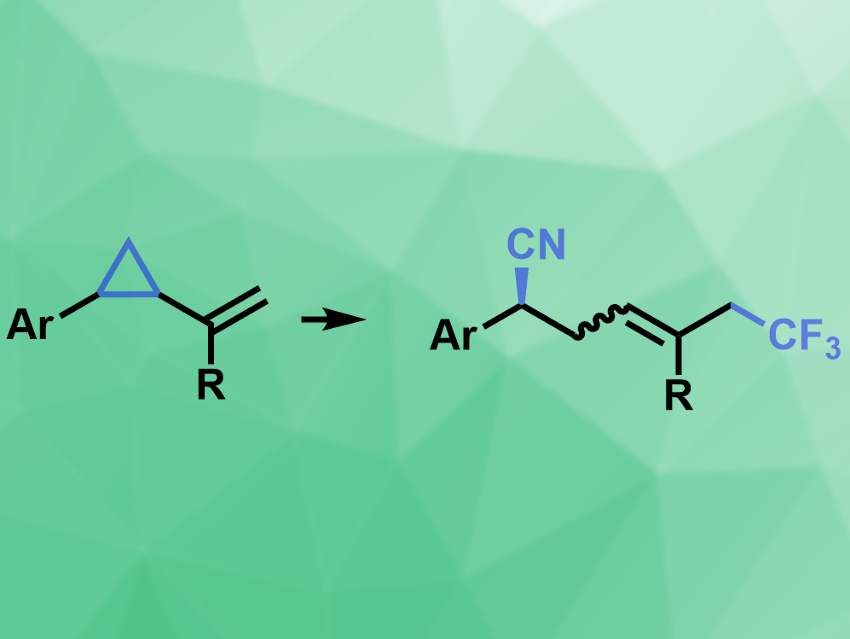
Xi-Sheng Wang, University of Science and Technology of China, Hefei, and colleagues have developed a copper-catalyzed enantioselective method for the 1,5-cyanotrifluoromethylation of vinylcyclopropanes (pictured). The team used Togni’s reagent as a source of ·CF3 radicals, Cu(acac)2 (acac = acetylacetone) as a catalyst together with a chiral bisoxazoline ligand, and trimethylsilyl cyanide (TMSCN) as a cyano source to convert vinylcyclopropanes bearing different (hetero)arenes into the desired products.
The reaction proceeds at room temperature and provides good to excellent yields and enantioselectivities. The team proposes a Cu(I)/Cu(III)-catalyzed reaction mechanism that involves the reaction of the ·CF3 radical with the alkene, followed by ring-opening of the cyclopropane, and the remote functionalization with the cyano reagent mediated by the chiral copper complex.
- Enantioselective Copper-Catalyzed 1,5-Cyanotrifluoromethylation of Vinylcyclopropanes,
Zi-Qi Zhang, Xiang-Yu Meng, Jie Sheng, Quan Lan, Xi-Sheng Wang,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b03012


![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
