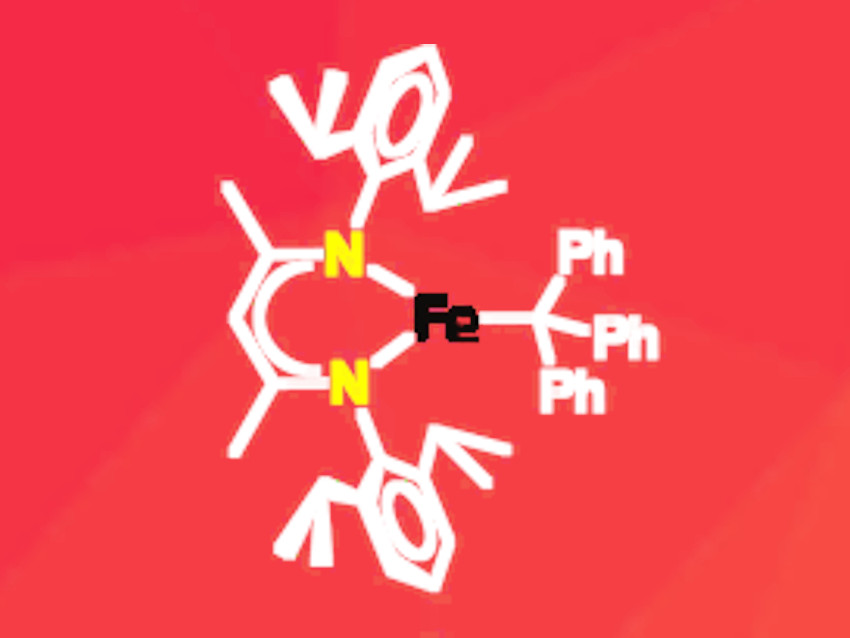
Iron is a metal with a rich coordination chemistry due to its wide variety of easily accessible redox and spin states. The formal electron count at the metal center and on the ligands is usually determined by applying the octet rule. However, the experimental assignment of the actual electron distribution within a metal complex often proves to be more challenging.
Kyle M. Lancaster, Cornell University, Ithaca, NY, USA, Patrick L. Holland, Yale University, New Haven, CT, USA, and colleagues have synthesized the first iron–trityl (CPh3) complex (pictured) and analyzed its electron distribution. The complex is stabilized by a β-diketiminate ligand with two bulky aryl groups. These ligands form stable complexes with iron in different oxidation states. The researchers synthesized the complex by reacting a binuclear iron precursor carrying the bulky β-diketiminate ligand with the trityl dimer in diethyl ether.
The product was characterized by NMR and Mössbauer spectroscopy as well as X-ray crystallography. Density functional theory (DFT) and multireference configuration interaction (MRCI) calculations were carried out to determine the electron distribution. The results indicate that the complex can be described as an iron(II) species with a “masked” radical character in the trityl ligand. This has been confirmed by the experimental finding that the complex releases a trityl radical when ligands such as benzophenone and phenylacetylene are added. The free radical is then stabilized by dimerization.
The iron(II) oxidation state formally implies radical character on the ligand. However, the researchers emphasize that the mixing of metal and ligand orbitals is so extensive that little actual radical character is present on the ligand within the complex.
- Masked Radicals: Iron Complexes of Trityl, Benzophenone, and Phenylacetylene,
K. Cory MacLeod, Ida M. DiMucci, Edward P. Zovinka, Sean F. McWilliams, Brandon Q. Mercado, Kyle M. Lancaster, Patrick L. Holland,
Organometallics 2019.
https://doi.org/10.1021/acs.organomet.9b00534