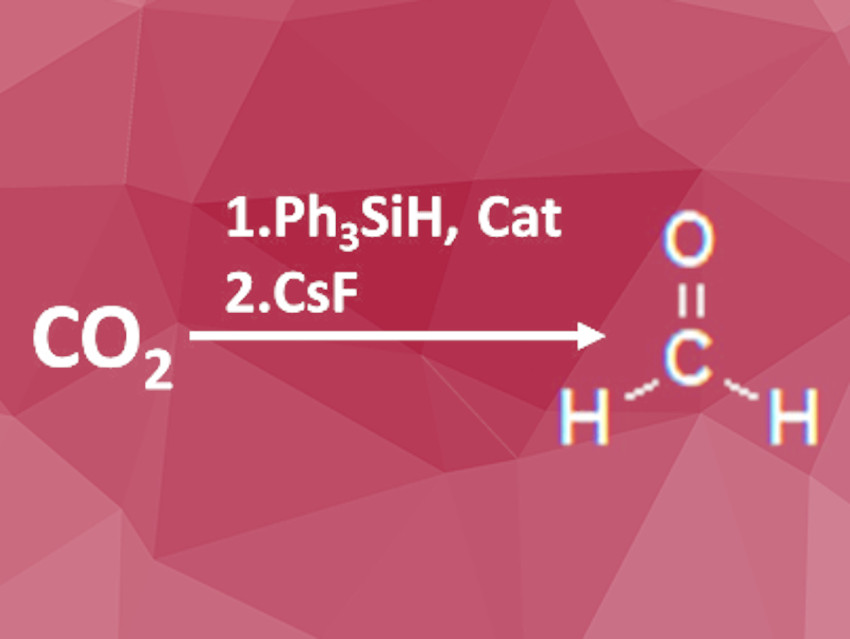
While CO2 is often produced as an undesired side product in industrial processes, formaldehyde is an important building block for the synthesis of more complex molecules. Converting CO2 to formaldehyde is, therefore, a highly value-adding process. Due to its high stability, chemical transformations of CO2 are often difficult to achieve, however.
Gerard Parkin, Columbia University, New York, NY, UA, and colleagues have developed a selective reduction process from CO2 to formaldehyde via the bis(silyl)acetal. CO2 and two equivalents of triphenylsilane were reacted to form the bis(silyl)acetal. This reaction was catalyzed by a mixture of [TismPriBenz]MgX (X = H, Me), a magnesium complex carrying bulky, silicon-containing ligands, and tris(pentafluorophenyl)borane. The resulting silylated intermediate was isolated and the structure determined by X-ray-diffraction.
H2C(OSiPh3) was also successfully used as a C1-building block in various reactions such as, for example, the Pictet-Spengler reaction or hydrazone formation. The compound was air-stable at room temperature but free formaldehyde was generated by hydrolysis at elevated temperatures or room temperature under acid catalysis. The addition of cesium fluoride led to the release of formaldehyde at room temperature as well. The researchers also showed that this reaction can be used to selectively generate isotopologues by using 13CO2 or SiPh3D.
According to the researchers, the new procedure allows the direct reduction of CO2 to formaldehyde at room temperature without prior reduction to methanol and re-oxidation as required for previously applied processes. This reaction releases monomeric formaldehyde in aprotic solvents making it available for further reactions. The intermediate bis(silyl)acetal is stable at room temperature and can be used as a formaldehyde surrogate for a wide range of reactions.
- Selective Conversion of Carbon Dioxide to Formaldehyde via a Bis(silyl)acetal: Incorporation of Isotopically Labeled C1 Moieties Derived from Carbon Dioxide into Organic Molecules,
Michael Rauch, Zack Strater, Gerard Parkin,
Journal of the American Chemical Society 2019.
https://doi.org/10.1021/jacs.9b08342