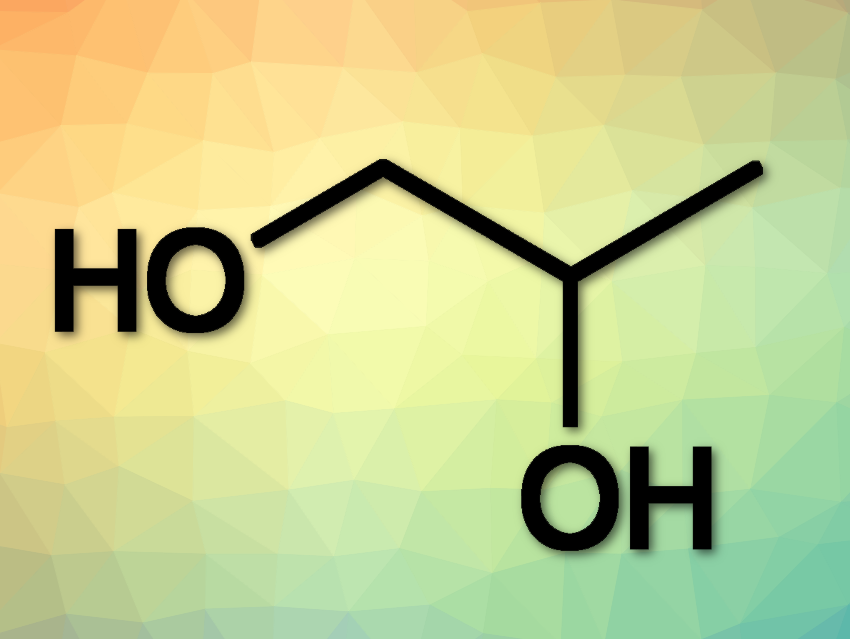
Dow and Evonik plan to bring a unique method for directly synthesizing propylene glycol (PG) from propylene and hydrogen peroxide to market maturity. The method is named HYPROSYN™. The key element is a catalytic system developed by Evonik researchers which allows for the direct synthesis of PG from propylene and hydrogen peroxide, in a process offering high yield and comparatively low energy consumption.
A pilot plant is to be constructed at the Evonik site in Hanau, Germany, by the end of 2020, with large-scale technical implementation to follow a few years later. Evonik is one of the world’s largest manufacturers of hydrogen peroxide.
Some 1.9 million metric tons of propylene glycol were used worldwide in 2018. The molecule is used in the production of polyester resins and as a de-icing agent. It is also an important food additive and serves as a humectant and co-surfactant in many products in the home and personal care market. Dow is the largest producer of propylene glycol worldwide.
In the traditional process, propylene oxide is converted to propylene glycol using water. According to the companies, the HYPROSYN™ technology offers several advantages over this process:
- It is expected to consume significantly less energy while providing a higher yield.
- It combines all reaction steps in a single reactor, eliminating the need for investment in additional propylene oxide capacity. Existing propylene glycol plants can be retrofitted with little effort.
- Only hydrogen peroxide and propylene are processed as feedstock, increasing flexibility and lowering total investment cost.
- Dow Inc., Midland, MI, USA
- Evonik Industries AG, Essen, Germany