The so-called “polysulfide shuttle effect” is a problem for lithium-sulfur batteries. The theoretical high performance of these cells is severely decreased in practice due to this effect. The sulfur or lithium-sulfide cathode forms intermediate lithium polysulfides, which dissolve in the electrolyte and “shuttle” from cathode to anode. There, they deposit an insoluble sulfide layer on the anode. The result is a loss of cathode material and a decreased cycle life of the battery.
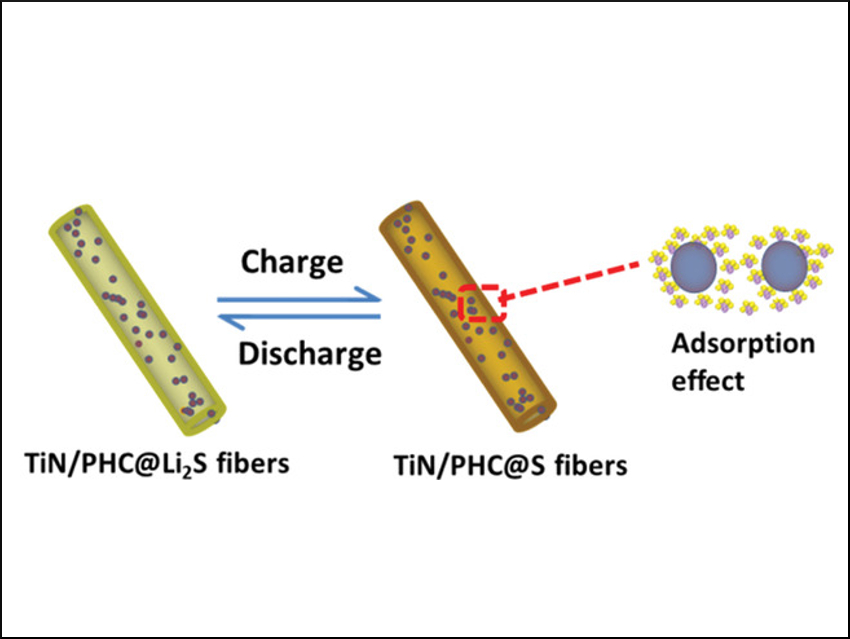
Lixia Yuan, Yunhui Huang, Huazhong University of Science and Technology, Wuhan, China, and colleagues have developed an approach to decrease this shuttle effect. The researchers made a composite cathode from a free-standing matrix of interconnected parallel hollow carbon (PHC) containing titanium nitride (TiN) nanoparticles. The TiN/PHC material was fabricated via electrospinning and subsequent high-temperature pyrolysis. Then the pores of the material were loaded via dip-coating with the active cathode material, i.e., lithium-sulfide nanoparticles.
The resulting composite cathode shows good mechanical properties. Its electronic conductivity is improved by including PHC and titanium nitride. Most importantly, titanium nitride acts as a “polysulfide immobilizer” because lithium polysulfides can be strongly adsorbed on its surface. A full cell with the composite cathode and a silicon-metal anode shows significantly improved reversible capacity and cyclability compared to the same setup without titanium nitride. This work shows that by smart cathode design, the polysulfide shuttle effect is decreased and a longer battery life and a better performance can be achieved.
- Advanced Li2S/Si Full Battery Enabled by TiN Polysulfide Immobilizer,
Zhangxiang Hao, Jie Chen, Lixia Yuan, Qiming Bing, Jingyao Liu, Weilun Chen, Zhen Li, Feng Ryan Wang, Yunhui Huang,
Small 2019.
https://doi.org/10.1002/smll.201902377