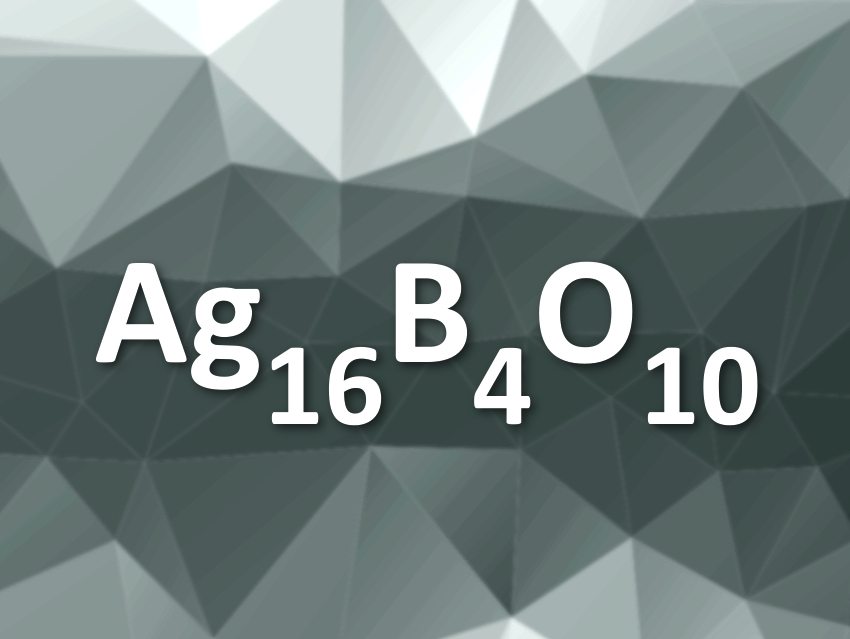Silver usually occurs in the Ag(I) oxidation state in its compounds. It does, however, not always act like simple alkali metal ions, which also carry a single positive charge. Congling Yin, Max Planck Institute for Solid State Research, Stuttgart, Germany, and Guilin University of Technology, China, Martin Jansen, Max Planck Institute for Solid State Research, and colleagues have synthesized a new ternary silver oxide, Ag16B4O10.
The compound was obtained in crystalline form via a hydrothermal synthesis starting from elemental Ag and H3BO3. Its structure was analyzed using X-ray diffraction, its electric and magnetic properties were tested, and its bonding situation was studied using density functional theory (DFT) calculations.
B4O108– is a borate cage anion. This anion is rare, but has a standard bonding situation. It is isolated in the structure of Ag16B4O10, i.e., separated from neighboring anions by octahedrons made up of silver atoms. This has allowed the team to determine the precise structure of this anion for the first time.
The charge of the anion leaves eight positive charges to be divided by 16 silver atoms. This means that Ag has a formal oxidation state of +0.5, or that eight excess electrons have to be accommodated per formula unit compared to a “normal” Ag(I) compound. However, these electrons do not just move freely through the silver substructure: The compound is not metallic, but a semiconductor. This means that the excess electrons are localized. It also is diamagnetic, i.e., the excess electrons are paired.
The team found tetrahedral arrangements of silver atoms in the structure that have some unusually short Ag–Ag bond distances. This could be explained by additional covalent interactions in these tetrahedra that are formed by the excess electron pairs. This hypothesis was confirmed by DFT calculations. According to the researchers, this confirms that some silver compounds can violate the usual valence rules by accommodating excess electron pairs.
- Uncommon structural and bonding properties in Ag16B4O10,
Anton Kovalevskiy, Congling Yin, Jürgen Nuss, Ulrich Wedig, Martin Jansen,
Chem. Sci. 2020.
https://doi.org/10.1039/c9sc05185f