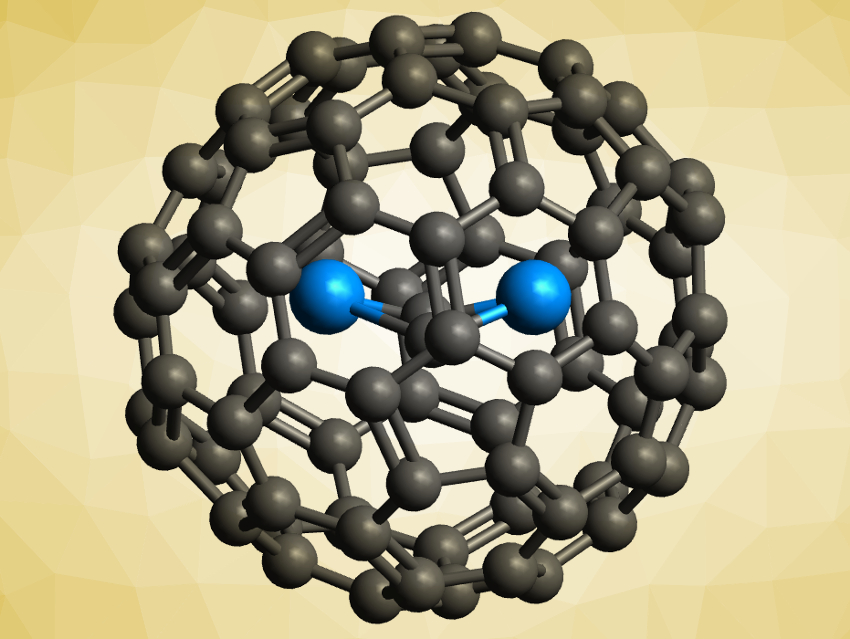
Fullerenes can be used to encapsulate actinide atoms and clusters. The resulting actinide endohedral metallofullerenes (EMFs) have strong metal–cage interactions, which can stabilize both unusual fullerenes and unprecedented actinide species.
Jochen Autschbach, University at Buffalo, New York, USA, Ning Chen, Soochow University, Suzhou, China, and colleagues have synthesized two previously unknown uranium carbide cluster EMFs, U2C2@C80 and U2C2@C78 (pictured). The team vaporized graphite rods packed with U3O8 and graphite powder in an arcing chamber under a helium atmosphere and extracted the products from the resulting soot. The EMFs were characterized using mass spectrometry (MS), single-crystal X-ray crystallography, X-ray absorption spectroscopy (XAS), and UV–Vis, Raman, infrared (IR), and NMR spectroscopy. The team also performed quantum-chemical calculations to investigate the bonding in the compounds.
The team found that the U2C2 clusters inside the fullerenes have a butterfly shape, with the two uranium atoms bridged by a C≡C unit—the first examples of a uranium carbide cluster with this structure. The results of the calculations indicate that the formal oxidation state of uranium in the compounds is +4 and the U–C bonds are mostly ionic with some covalent character. Both of these properties are different from previously reported uranium cluster fullerenes, which shows that uranium clusters can realize very different bonding situations inside fullerene cages.
- Diuranium(IV) Carbide Cluster U2C2 Stabilized Inside Fullerene Cages,
Jiaxin Zhuang, Laura Abella, Dumitru-Claudiu Sergentu, Yang-Rong Yao, Meihe Jin, Wei Yang, Xingxing Zhang, Xiaomeng Li, Duo Zhang, Yiming Zhao, Xiaohong Li, Shuao Wang, Luis Echegoyen, Jochen Autschbach, Ning Chen,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b10247