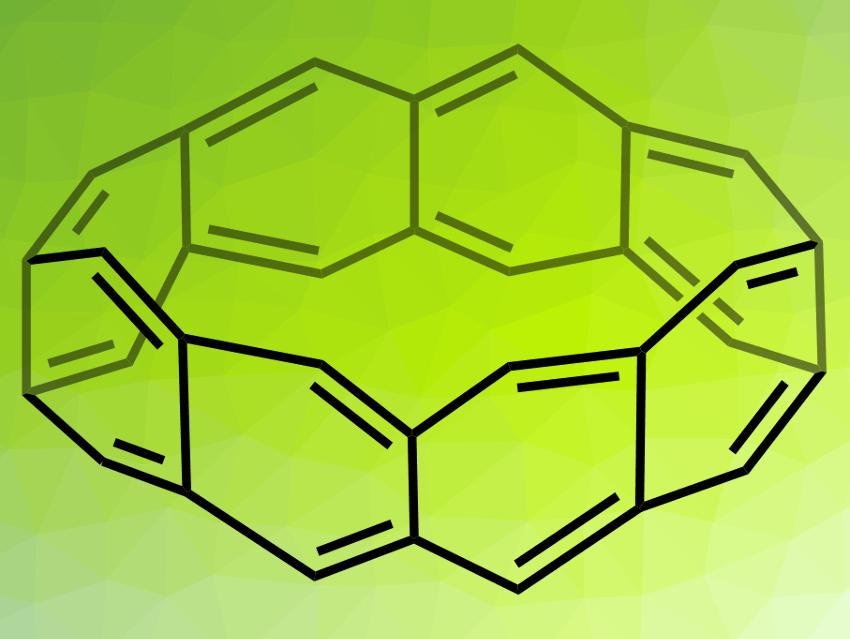
Belt-shaped hydrocarbons, such as the fully conjugated beltarenes (example pictured), could have applications, e.g., in nanochemistry or supramolecular chemistry. However, their synthesis is very challenging. The unsaturated beltarenes, in particular, are unstable due to their high strain, while partially saturated derivatives are slightly easier to access.
Mei-Xiang Wang, Tsinghua University, Beijing, China, and colleagues have developed a general approach to hydrocarbon belts and their derivatives and observed the fully conjugated belt[8]arene for the first time. The team started from resorcin[4]arene derivatives, which were converted to octohydrobelt[8]arenes using successive intramolecular Friedel–Crafts alkylations in a one-pot reaction. The resulting products were subjected to an oxidative aromatization using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ). The team obtained derivatives of tetrahydrobelt[8]arenes and fully conjugated belt[8]arenes, such as tetrahydrobelt[8]arene-DDQ2 and belt[8]arene-DDQ4.
The researchers prepared belt[8]arene (pictured) from the belt[8]arene-DDQ4 adduct via a retro-Diels–Alder reaction under laser irradiation. The identity of the product was confirmed by matrix-assisted laser desorption/ionization (MALDI) mass spectroscopy. According to the team, the formation of beltarenes had not been reported before.
- Toward the Synthesis of a Highly Strained Hydrocarbon Belt,
Tan-Hao Shi, Qing-Hui Guo, Shuo Tong, Mei-Xiang Wang,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c00112