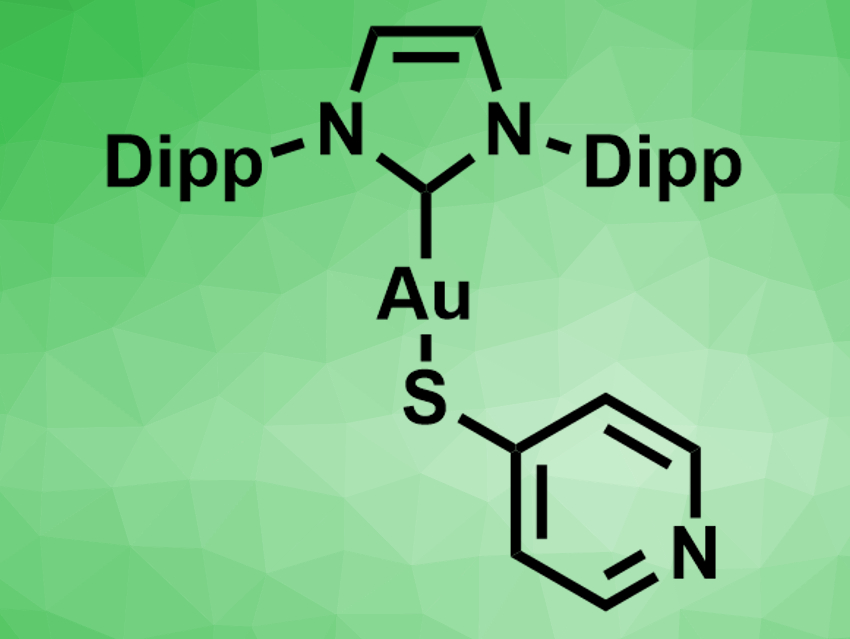
Gold complexes with N-heterocyclic carbene (NHC) ligands have been used in catalysis and medicinal chemistry. Often, complexes of the type (NHC)Au–X (X = halide) have been used. Replacing the halide ligand with a sulfur-based ligand can lead to more stable complexes with new functions. Ligands with sulfur/nitrogen heterocycles, such as mercaptopyridines or mercaptothiazolines, have also been used. In these complexes, the sulfur atom binds to the gold center and the nitrogen atom can potentially coordinate a second metal.
Daniel Mendoza-Espinosa, Autonomous University of the State of Hidalgo, Mineral de la Reforma, Mexico, and colleagues have prepared (NHC)AuI–mercaptopyridine complexes (example pictured, Dipp = 2,6-diisopropylphenyl), as well as related heteronuclear Au/Pd complexes in which the pyridine nitrogen coordinates a palladium allyl chloride unit. The team prepared the Au(I) complexes from the corresponding chloride or iodide complexes via ligand exchange with either 2- or 4-mercaptopyridine and excess KOH in tetrahydrofuran (THF). The products were characterized using 1H and 13C NMR spectroscopy, as well as single-crystal X-ray diffraction. The complexes show the expected linear (NHC)–Au–S coordination.
The heterometallic Au/Pd complexes were prepared from the synthesized gold complexes by the reaction with a palladium allyl chloride dimer in THF at 60 °C. The resulting bimetallic complexes might be useful in catalysis. The team also reacted the gold complexes with B(C6F5)3 in the presence of ammonium fluoride and found that the pyridine nitrogen can form adducts of the type Npy–H–F–B(C6F5)3. These complexes could be useful for the activation of small molecules.
- Synthesis and Reactivity of (NHC)AuI–Mercaptopyridine Complexes,
R. Evelyn Cordero-Rivera, David Rendón-Nava, Carlos Ángel-Jijón, Oscar R. Suárez-Castillo, Daniel Mendoza-Espinosa,
Organometallics 2020.
https://doi.org/10.1021/acs.organomet.0c00118