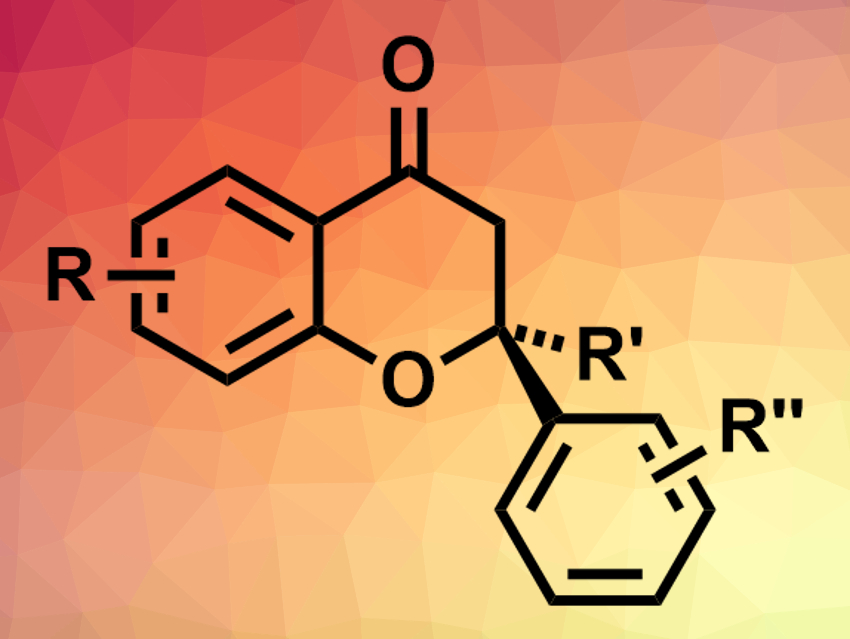
Chiral chromanones (example pictured above) are often found in bioactive natural products. Existing methods for their enantioselective preparation are generally aimed at trisubstituted stereocenters at the C2 position, not the tetrasubstituted derivatives.
Brian M. Stoltz, California Institute of Technology, Pasadena, USA, Sukwon Hong, Gwangju Institute of Science and Technology, Republic of Korea, and colleagues have developed a palladium-catalyzed asymmetric conjugate addition of arylboronic acids to 2-substituted chromones using newly developed chiral pyridine-dihydroisoquinoline ligands (PyDHIQ, pictured below). This reaction gives chiral chromanones with tetrasubstituted stereocenters (pictured above).
 The team used palladium(II) trifluoroacetate as the catalyst, PyDHIQ as the ligand, water as the solvent, and NH4PF6 as an additive. The reaction was performed under an oxygen atmosphere at 70°C. Under these conditions, a range of substituted aryl boronic acids was reacted with different chromones to give the desired chromanones in moderate to excellent yields with enantioselectivities of up to 99 % ee.
The team used palladium(II) trifluoroacetate as the catalyst, PyDHIQ as the ligand, water as the solvent, and NH4PF6 as an additive. The reaction was performed under an oxygen atmosphere at 70°C. Under these conditions, a range of substituted aryl boronic acids was reacted with different chromones to give the desired chromanones in moderate to excellent yields with enantioselectivities of up to 99 % ee.
- Catalytic Enantioselective Synthesis of Tetrasubstituted Chromanones via Palladium-Catalyzed Asymmetric Conjugate Arylation Using Chiral Pyridine-Dihydroisoquinoline Ligands,
Doohyun Baek, Huijeong Ryu, Ji Yeon Ryu, Junseong Lee, Brian M. Stoltz, Sukwon Hong,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc00412j


![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
