The current worldwide outbreak of the respiratory disease COVID-19 is caused by the coronavirus SARS-CoV-2. Antiviral medication to treat this disease is urgently needed. The virus’s main protease Mpro (3CLpro) is a possible target for these drugs. Inhibiting this essential enzyme can prevent the replication of the virus. There is no analogous enzyme in humans, which makes Mpro a good drug target.
Lei-Ke Zhang, Wuhan Institute of Virology, Chinese Academy of Sciences, Yechun Xu, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Haitao Yang, ShanghaiTech University, China, Hong Liu, Shanghai Institute of Materia Medica, China Pharmaceutical University, Nanjing, and Nanjing University of Chinese Medicine, and colleagues have designed two new antiviral drug candidates that target SARS-CoV-2 Mpro. The team analyzed the structure of the active site of the enzyme to design these inhibitors.
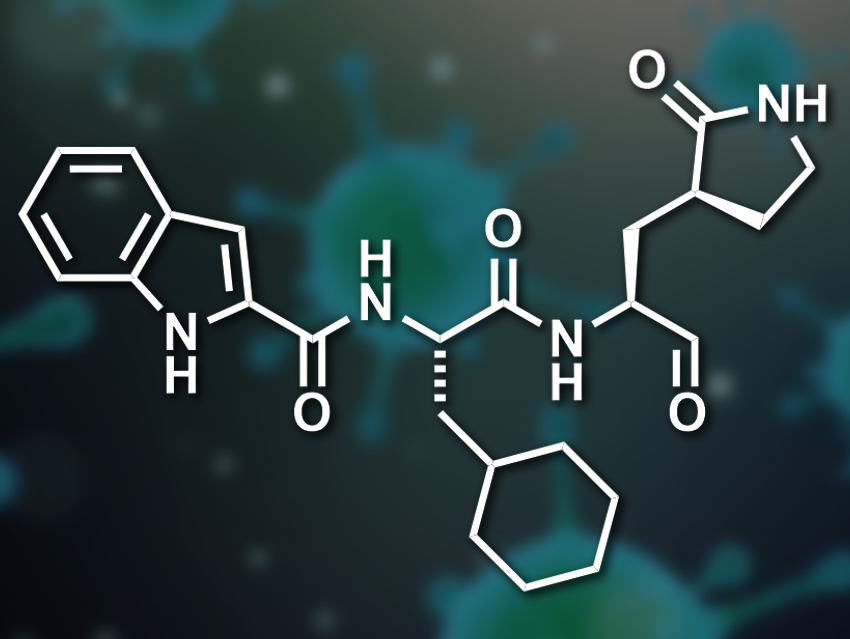
The active site has four substrate-binding pockets, one with a cysteine residue. The researchers proposed the inclusion of an aldehyde functional group in the drug candidates to form a covalent bond with this cysteine. For the second binding site, a γ-lactam ring had shown promise in other reported SARS-CoV Mpro inhibitors. For the larger third pocket, the team included either a cyclohexyl- or a 3-fluorophenyl group. For the fourth binding site, an indole group that can form hydrogen bonds with the enzyme was added.
The resulting rationally designed drug candidates were synthesized and tested in enzymatic assays. The team found that both of the candidates (with either a cyclohexyl- or a 3-fluorophenyl group) have a high SARS-CoV-2 Mpro inhibition activity. The activity was slightly higher for the candidate with the cyclohexyl substituent (pictured). The researchers determined a high-resolution crystal structure of a complex between this drug candidate and the enzyme at a resolution of 1.5 Å. They found that the compound sits in the enzyme’s substrate binding pocket and that the aldehyde’s carbon atom is covalently bound to the targeted cysteine.
Both drug candidates showed low toxicity and promising pharmacokinetic properties in animals. According to the team, the cyclohexyl-substituted compound could be a good candidate for further clinical studies.
- Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease,
Wenhao Dai, Bing Zhang, Haixia Su, Jian Li, Yao Zhao, Xiong Xie, Zhenming Jin, Fengjiang Liu, Chunpu Li, You Li, Fang Bai, Haofeng Wang, Xi Cheng, Xiaobo Cen, Shulei Hu, Xiuna Yang, Jiang Wang, Xiang Liu, Gengfu Xiao, Hualiang Jiang, Zihe Rao, Lei-Ke Zhang, Yechun Xu, Haitao Yang, Hong Liu,
Science 2020.
https://doi.org/10.1126/science.abb4489
Also of Interest
- Collection: SARS-CoV-2 Virus
What we know about the new coronavirus and COVID-19 - LitCovid
Curated literature hub for tracking up-to-date scientific information about COVID-19 - Many publishers and other entities have signed a joint statement to ensure that COVID-19 research findings and data are shared rapidly and openly