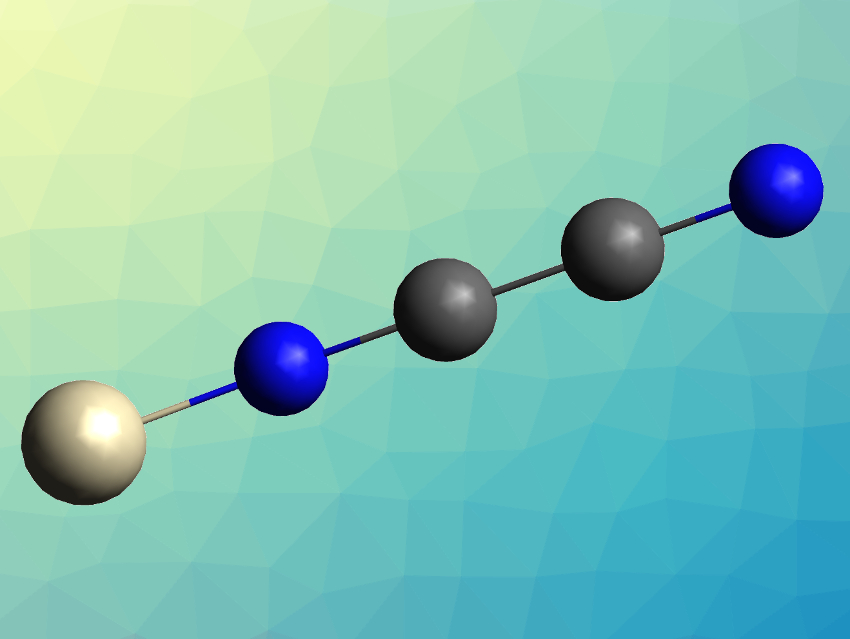
Cyanogen (NCCN) is a Lewis base and can bind to metal centers, either as an “end-on”, nitrogen-bound ligand (pictured) or as a “side-on” ligand bound via both carbon and nitrogen atoms. Transition metal–cyanogen complexes have not been extensively studied so far, and had only been known for the metals Rh, Ru, Ni, Cu, Ag, Zn, and Cd.
Yu Gong, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, and University of Virginia, Charlottesville, USA, and colleagues have synthesized the cyanogen complexes [Ir(NCCN)], [Pd(NCCN)], and [Pt(NCCN)] in argon matrices at 4 K. The team used laser ablation to generate Ir, Pd, or Pt atoms from a metal target, and synthesized cyanogen via the thermal decomposition of AgCN at 360–380 °C. The metal atoms and cyanogen were reacted in excess argon.
The products were characterized using infrared (IR) spectroscopy and theoretically studied using density functional theory (DFT) calculations. The team found that in all three cases, the cyanogen ligand is bound end-on to the metal by a nitrogen atom, forming a linear complex. The bond lengths in the cyanogen ligands were elongated by 0.01–0.015 Å for the C–N bonds and shortened by 0.013–0.02 Å for the C–C bonds compared with the free ligand. The team attributes this to electronic back-donation from metal to ligand, which destabilizes the C–N bond and stabilizes the C–C bond. This was confirmed by the computational results.
- End-On Cyanogen Complexes of Iridium, Palladium, and Platinum,
Xiuting Chen, Zhixin Xiong, Lester Andrews, Yu Gong,
Inorg. Chem. 2020.
https://doi.org/10.1021/acs.inorgchem.0c00582