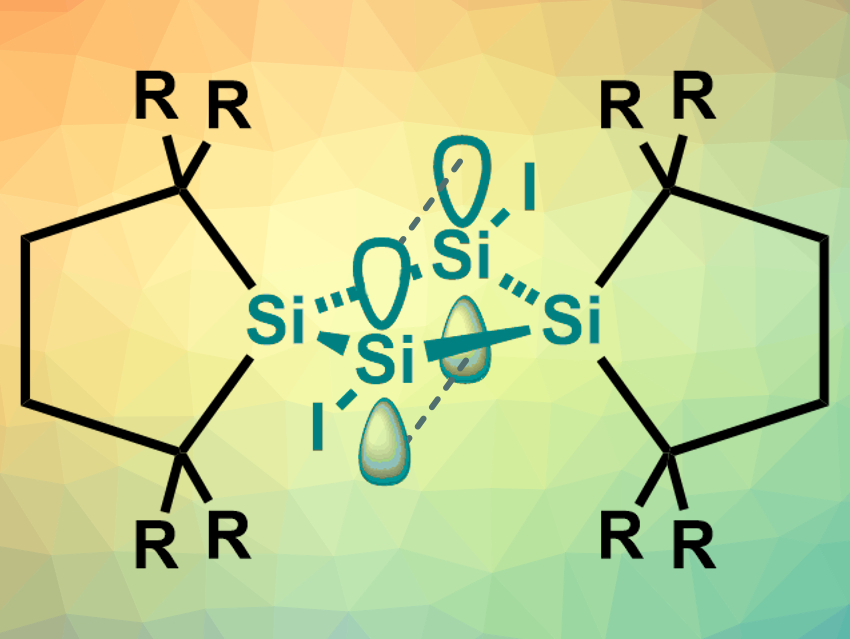
Multiple bonds, such as those in alkenes or alkynes, consist of a σ-bond accompanied by one or more π-bonds. Compounds that contain a π-bond without an underlying σ-bond, i.e., a π-type single bond, are rare and generally unstable. However, some of them can be stabilized by tuning their electronic and geometric structure.
Takumi Nukazawa and Takeaki Iwamoto, Tohoku University, Sendai, Japan, have synthesized an isolable compound with a π-type single bond, 1,3-diiodotetrasilabicyclo[1.1.0]butane (pictured, R = SiMe3). The team started from tetrasilabicyclo[1.1.0]but-1(3)-ene, which contains an Si═Si bond across the central ring. This substrate was reacted with 1,2-diiodoethane in toluene at 0 °C to give the desired product in a yield of 35 %. The product was obtained in the form of air-sensitive orange-red crystals, which were recrystallized in benzene and then characterized using X-ray diffraction, NMR spectroscopy, mass spectrometry, and elemental analysis.
The team found that the structure contains a planar central Si4 ring. The high symmetry of the compound was confirmed by the 1H and 29Si NMR spectra. The team performed density functional theory (DFT) calculations to investigate the bonding between the “bridgehead” silicon atoms. The results indicate that interactions between p orbitals of the two Si atoms form a bridgehead π-type single bond (pictured). The team attributes the stability of this unusual structure to the presence of sterically demanding substituents.
- An Isolable Tetrasilicon Analogue of a Planar Bicyclo[1.1.0]butane with π-Type Single-Bonding Character,
Takumi Nukazawa, Takeaki Iwamoto,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c03874