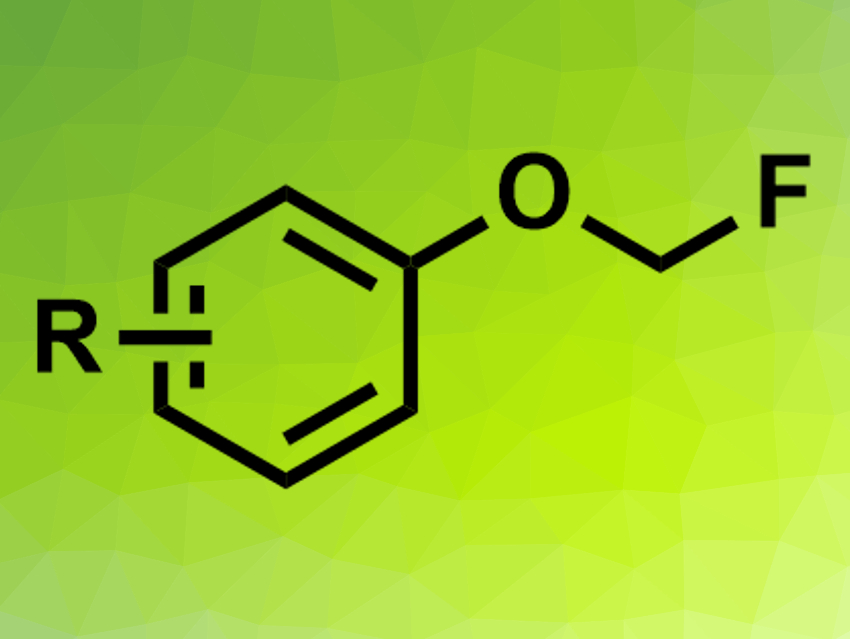
Fluoromethyl aryl ethers (pictured) have potential applications in, e.g., agrochemistry and pharmaceutical chemistry. Fluorine atoms can often positively impact the properties of drug candidates, for example. Fluoromethyl aryl ethers can be synthesized from phenols using FCH2Cl, but this reagent is a greenhouse gas and requires a multi-step synthesis. Fluorodecarboxylations, i.e., reactions in which carboxylates are converted to fluoromethyl ethers under loss of CO2, could be an alternative. However, existing methods for this type of reaction require metal catalysts or an excess of oxidizing and fluorinating agents.
Siegfried R. Waldvogel, University of Mainz, Germany, and colleagues have developed a sustainable, metal-free, electrochemical fluorodecarboxylation of aryloxyacetic acids (Ar–O–CH2–COOH) to give fluoromethyl aryl ethers. The team used a variety of (hetero-)aryloxyacetic acids as substrates, which were electrolyzed using a constant current in an undivided cell with graphene electrodes. The researchers used triethylamine pentahydrofluoride (Et3N·5HF) as fluoride source, 2,4,6-trimethylpyridine as a base, and CH2Cl2 as a solvent.
The desired fluoromethyl (hetero-)aryl ethers were obtained in moderate to good yields. The utility of the method was demonstrated by successful fluorodecarboxylations of derivatives of natural products such as thymol and vitamin E. According to the team, the method is readily scalable.
- Metal-free electrochemical fluorodecarboxylation of aryloxyacetic acids to fluoromethyl aryl ethers,
Michael Berger, John D. Herszman, Yuji Kurimoto, Goswinus H. M. de Kruijff, Aaron Schüll, Sven Rufc, Siegfried R. Waldvogel,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc02417a