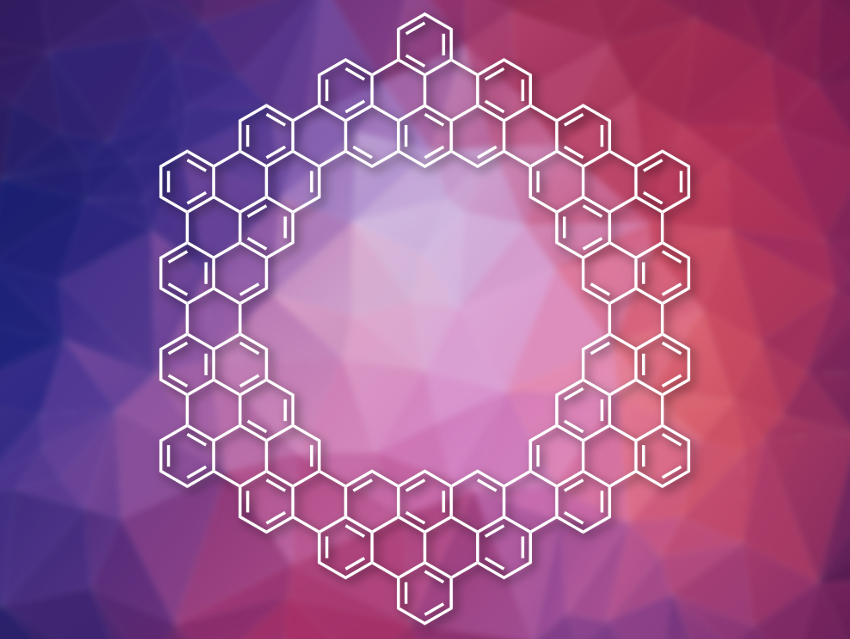
Coronoids are circular polycyclic aromatic hydrocarbons with a central cavity. Kekulene (pictured on the right), a ring formed from benzene rings, is a simple example of this type of compound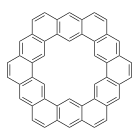 . Larger coronoids with more than one layer of benzene rings can also be considered to be porous nanographenes. These coronoids can be difficult to characterize due to their low solubility. On-surface synthesis can be used for the preparation of coronoids and, at the same time, allow their characterization using methods such as scanning tunneling microscopy (STM) or atomic force microscopy (AFM).
. Larger coronoids with more than one layer of benzene rings can also be considered to be porous nanographenes. These coronoids can be difficult to characterize due to their low solubility. On-surface synthesis can be used for the preparation of coronoids and, at the same time, allow their characterization using methods such as scanning tunneling microscopy (STM) or atomic force microscopy (AFM).
Marco Di Giovannantonio, Carlo A. Pignedoli, Swiss Federal Laboratories for Materials Science and Technology (Empa), Dübendorf, Klaus Müllen, Max Planck Institute for Polymer Research, Mainz, and University of Mainz, both Germany, Akimitsu Narita, Max Planck Institute for Polymer Research and Okinawa Institute of Science and Technology Graduate University, Japan, and colleagues have used an on-surface synthesis approach to synthesize C168 and C140 coronoids, which the team calls [6]coronoid (pictured) and [5]coronoid, respectively. The researchers used 5,9-dibromo-14-phenylbenzo[m]tetraphene as a precursor. It was heated on an Au(111) surface to first perform a dehalogenative aryl–aryl coupling (at 200 °C) and then a cyclodehydrogenation (at 380 °C) to give the desired coronoids.
Under highly diluted conditions, the team obtained yields of 30 % and 6 % for [5]- and [6]coronoid, respectively. Various chainlike structures and some macrocycles were formed as side products. The structures of [6]coronoid and [5]coronoid were investigated using AFM. The team found that [6]coronoid is planar on the gold surface and the size of its inner cavity is 1.4 nm. [5]Coronoid is nonplanar, with two of its inner edges appearing to be raised in STM and AFM images. The size of its pore is 1.1 nm. According to the researchers, the coronoids might be useful building blocks for larger nanoporous graphenes.
- Large-Cavity Coronoids with Different Inner and Outer Edge Structures,
Marco Di Giovannantonio, Xuelin Yao, Kristjan Eimre, José I. Urgel, Pascal Ruffieux, Carlo A. Pignedoli, Klaus Müllen, Roman Fasel, Akimitsu Narita,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c05268