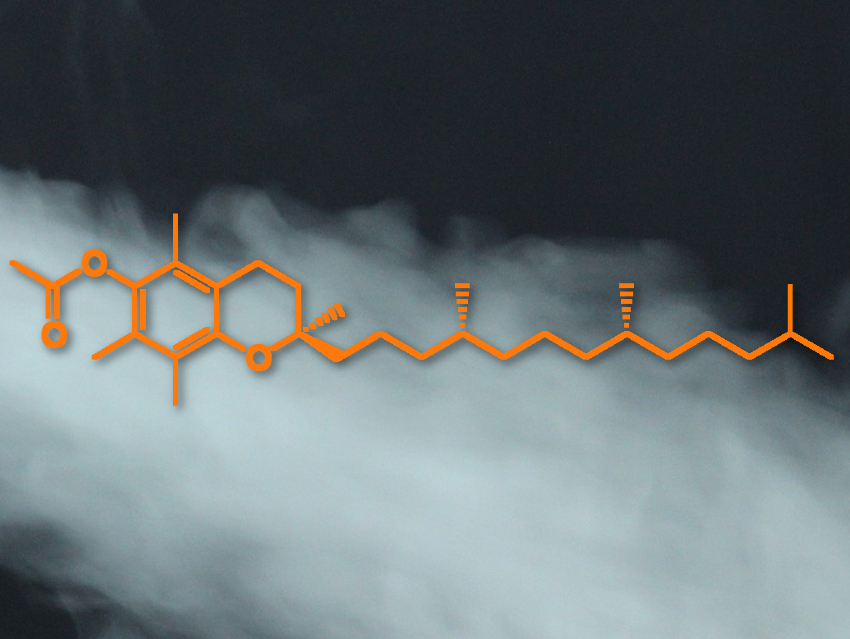
E-cigarettes are often marketed as a healthier alternative to tobacco products. However, e-cigarette- or vaping-associated lung injury (EVALI) is a dangerous disease that has affected thousands of mostly young patients. Studies have linked vitamin E acetate (pictured), found as an additive in some vaping liquids, to the disorder. Vitamin E acetate has been found in the lungs of most EVALI patients but not in healthy controls.
This harmful effect might be based on an interaction with the so-called “pulmonary surfactant”, a liquid lining found deep in the lungs, in the alveoli. This liquid is important to reduce the surface tension in this part of the lungs so that the alveoli can easily inflate when someone inhales. It is not known exactly how the surfactant layer behaves when a person breathes in and out, but one hypothesis is that certain lipids get “squeezed out” when the alveoli contract, and then spread across the surface again when the alveoli expand.
Drew Marquardt, University of Windsor, Canada, and colleagues have investigated how vitamin E acetate could influence this process. The team added vitamin E acetate to two different lipid-bilayer models for pulmonary surfactant: one containing only the lipid 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), which is the primary component of the surfactant, and the other containing a mixture of the four major lipids in the fluid.
The team found that increasing the vitamin E acetate concentration increases the lipid-bilayer membranes’ fluidity and compressibility for both model surfactants. This suggests that, in the presence of the vaping additive, the lung surfactant monolayer could “squeeze out” lipids prematurely during exhalation, and thereby become unstable. However, the researchers note that the model system is simplified and, e.g., does not include the effect of surfactant proteins. Thus, further work will be needed to confirm this theory.
- A Mechanical Mechanism for Vitamin E Acetate in E-cigarette/Vaping-Associated Lung Injury,
Mitchell DiPasquale, Omotayo Gbadamosi, Michael H. L. Nguyen, Stuart R. Castillo, Brett W. Rickeard, Elizabeth G. Kelley, Michihiro Nagao, Drew Marquardt,
Chem. Res. Toxicol. 2020.
https://doi.org/10.1021/acs.chemrestox.0c00212