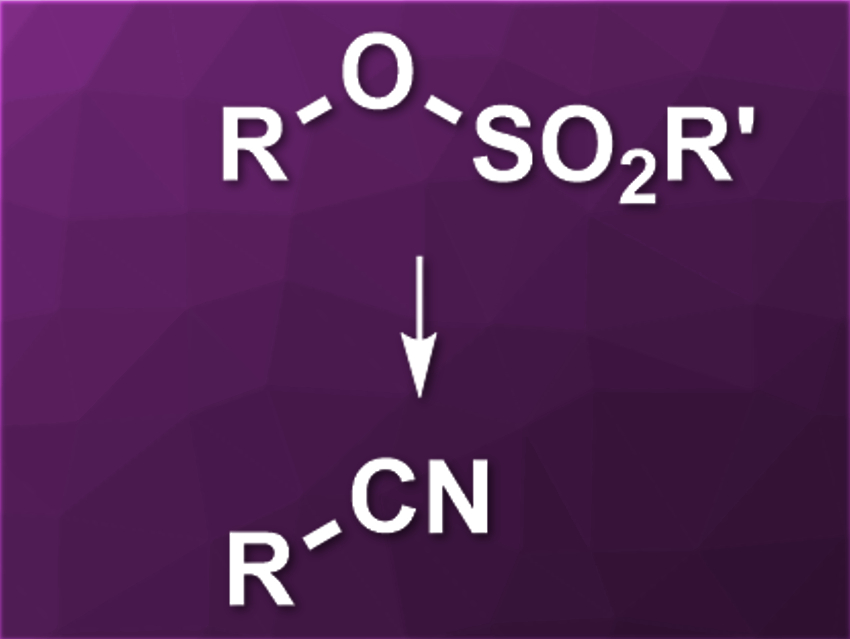
Transition-metal-catalyzed cross-coupling reactions are often used for the creation of C–C bonds. Aryl electrophiles are commonly used as coupling partners, while alkyl electrophiles are generally more difficult to use. Nitrile groups can be introduced using C–C coupling reactions and transition-metal-catalyzed cyanations of alkyl electrophiles would be useful. However, there are few examples of this type of reaction.
Yuanhong Liu, University of the Chinese Academy of Sciences, Shanghai, and colleagues have developed a nickel-catalyzed cyanation of unactivated alkyl sulfonates. The team used primary and secondary alkyl mesylates and reacted them with zinc cyanide in the presence of NiCl2·6 H2O as a catalyst and Xantphos as a ligand. Zinc powder was used as a reductant, 4-dimethylaminopyridine (DMAP) as a base/additive, n-Bu4NI as an additive, and acetonitrile as the solvent.
The desired alkyl nitriles were obtained in moderate to good yields. The reaction has a good functional group tolerance. According to the researchers, the reaction mechanism could involve an in–situ-generated alkyl iodide as an intermediate. The developed protocol might be useful, e.g., in pharmaceutical chemistry.
- Nickel-Catalyzed Cyanation of Unactivated Alkyl Sulfonates with Zn(CN)2,
Aiyou Xia, Peizhuo Lv, Xin Xie, Yuanhong Liu,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c02722

![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)

