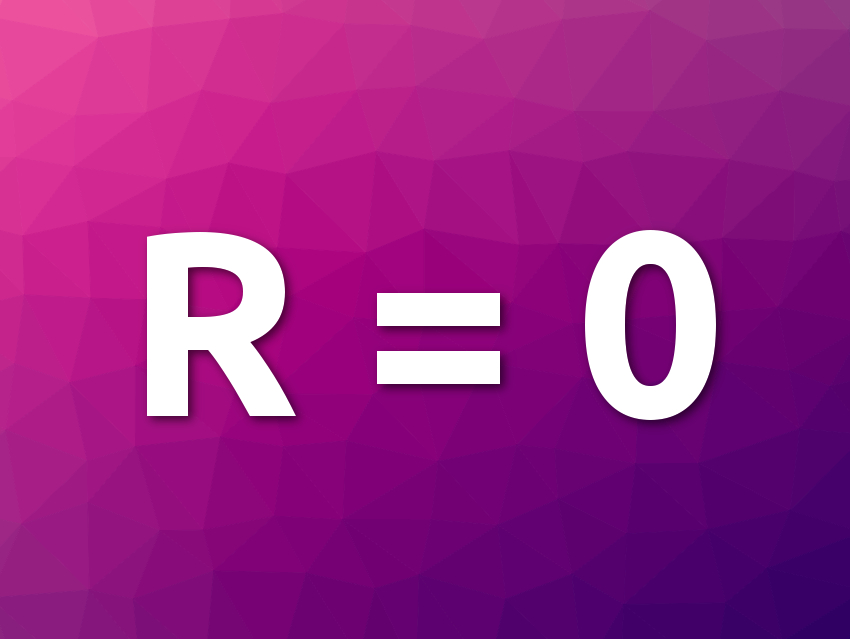Achieving room-temperature superconductivity is an important goal in physics and materials science. Materials that have zero electrical resistance at “normal” conditions would allow breakthroughs in several areas of technology. Pure hydrogen in a metallic state could theoretically be a promising superconductor, but achieving this state requires enormously high pressures. Using hydrogen-rich materials instead could allow researchers to achieve room-temperature superconductivity at lower pressures.
Ranga P. Dias, University of Rochester, NY, USA, and colleagues have developed a carbonaceous sulfur hydride system that has a maximum superconducting transition temperature of 287.7 ± 1.2 K (ca. 15°C or 58 °F), which makes it the first room-temperature superconductor. However, this transition temperature is achieved at a pressure of 267 ± 10 GPa—nowhere near “normal” conditions yet. The researchers used carbon, sulfur, and hydrogen as elemental precursors to prepare a hydrogen-rich carbonaceous sulfur hydride system, which was then photochemically transformed using laser light to give the superconductor.
The team observed superconductivity at pressures of 140–275 GPa. Above 220 GPa, the superconducting transition temperature rose sharply. According to the researchers, changing the system’s chemical composition could allow the preservation of room-temperature superconductivity at lower pressures.
- Room-temperature superconductivity in a carbonaceous sulfur hydride,
Elliot Snider, Nathan Dasenbrock-Gammon, Raymond McBride, Mathew Debessai, Hiranya Vindana, Kevin Vencatasamy, Keith V. Lawler, Ashkan Salamat, Ranga P. Dias,
Nature 2020, 586, 373–377.
https://doi.org/10.1038/s41586-020-2801-zUpdate (November 11, 2023)
The research article was retracted on September 26, 2022, after questions were raised regarding the manner in which the data in the paper were processed and analyzed. According to the editors, these processing issues undermine confidence in the published magnetic susceptibility data as a whole.Update (April 4, 2024)
A 10-month investigation, concluded on February 8, 2024, was conducted by an independent group of scientists recruited by the University of Rochester, NY, USA. The report found that Ranga Dias engaged in data fabrication, falsification, and plagiarism.Reference
Dan Garisto, Exclusive: official investigation reveals how superconductivity physicist faked blockbuster results – The confidential 124-page report from the University of Rochester, disclosed in a lawsuit, details the extent of Ranga Dias’s scientific misconduct, Nature April 6, 2024. https://doi.org/10.1038/d41586-024-00976-y