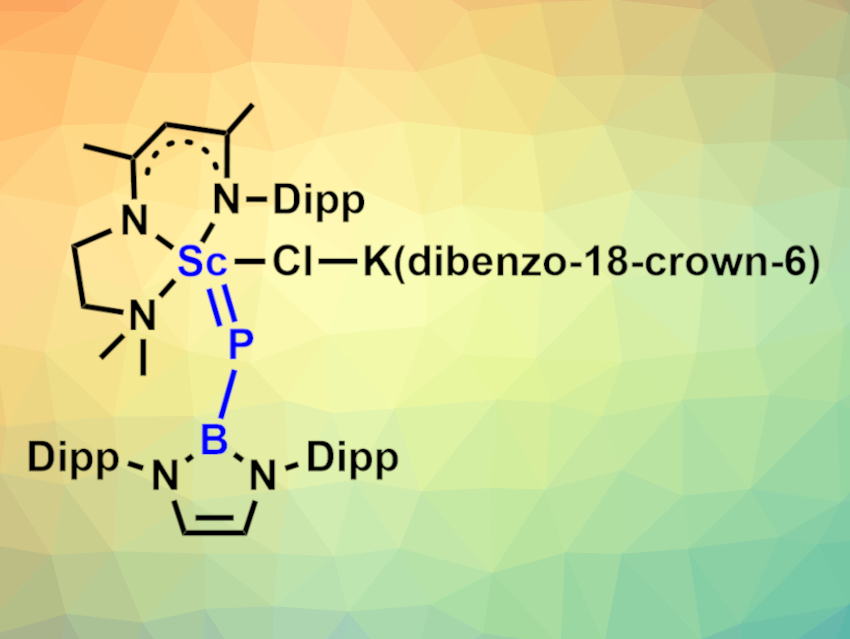
Transition-metal complexes with main-group ligands connected via a double or triple bond are interesting chemical species. For rare-earth metals, however, this type of complex is unusual. There have been a few examples of rare-earth-terminal imido, alkylidene, and oxo complexes, but no rare-earth-terminal phosphinidene complex had been reported so far.
Laurent Maron, Université Paul Sabatier, Toulouse, France, Yaofeng Chen, Shanghai Institute of Organic Chemistry, University of the Chinese Academy of Sciences, and colleagues have synthesized the first example of a rare-earth-terminal phosphinidene complex, a scandium boronylphosphinidene complex (pictured, Dipp = 2,6-(iPr)2C6H3). The team first prepared the potassium salt K[HPB{N(Dipp)CHCHN(Dipp)}] via the deprotonation of the corresponding boronylphosphine with KCH2(C6H5) in tetrahydrofuran (THF) and then treated it with [LSc(Me)Cl] (L = [MeC(NDipp)CHC(Me)NCH2CH2NMe2]−) in a THF/toluene mixture. This gave a heterometallic K/Sc phosphinidene complex in a yield of 43 %. Reacting this intermediate with dibenzo-18-crown-6 in toluene to bind the potassium ion than gave the desired scandium-terminal boronylphosphinidene complex in a yield of 83 %.
The team characterized the complex using single-crystal X-ray diffraction and found that it has a much shorter Sc–P bond length (2.381 Å) than a related scandium boronylphosphido complex. The results of density functional theory (DFT) calculations indicate that there is a strong Sc–P π interaction in the complex.
- Scandium-Terminal Boronylphosphinidene Complex,
Bin Feng, Li Xiang, Ambre Carpentier, Laurent Maron, Xuebing Leng, Yaofeng Chen,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c00148