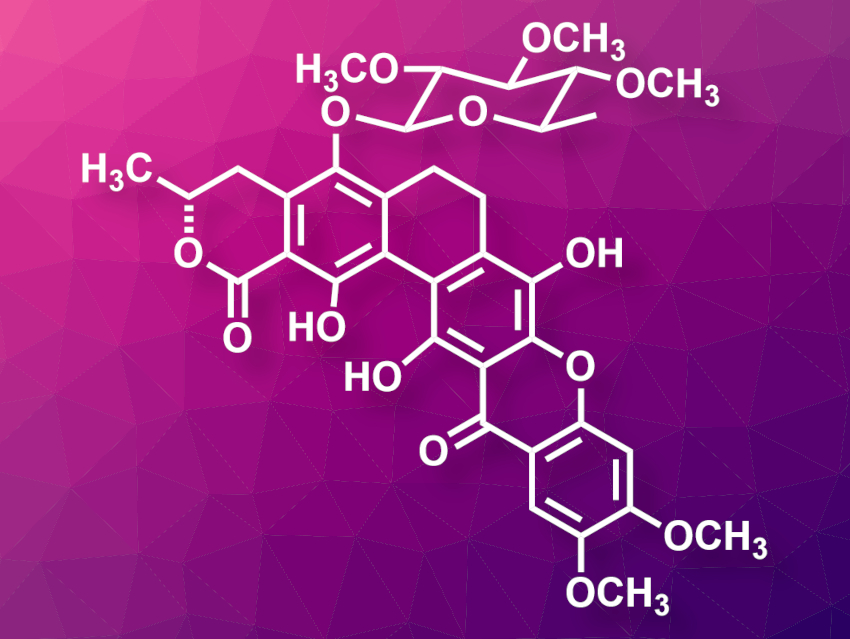
Calixanthomycin A (pictured) is a polycyclic natural product that was isolated from the bacterium Streptomyces albus. It has been shown to inhibit the growth of a human colon cancer cell line. The structure of calixanthomycin A has been determined based on spectroscopic data. However, the absolute configuration of the lactone in the A ring and the monosaccharide unit had not been assigned so far.
Xiaoli Zhao, Shuanhu Gao, East China Normal University, Shanghai, and colleagues have performed the first asymmetric total synthesis of calixanthomycin A and assigned the absolute configuration of the compound. The team used a modular synthesis approach in which the fragments for the A–B rings, for the D–E–F ring system, and the monosaccharide unit are synthesized separately and then connected to form the desired product. They started from 1,3-dibromo-2,5-dimethoxybenzene, which was converted to two enantiomers of the A–B ring system that were then functionalized with a terminal alkyne unit. The D–E–F ring system was prepared by coupling two highly functionalized benzene rings. Two enantiomers of the monosaccharide unit were synthesized from D– and L-glucose.
The prepared A–B and D–E–F ring fragments were then coupled, followed by a cyclization to close the C ring. Finally, the resulting hexacyclic intermediate was coupled with a monosaccharide fragment to obtain the desired product. The team then determined the absolute configuration of calixanthomycin A by comparing the spectral data and optical rotation of the synthesized stereoisomers with those of the natural product.
- Calixanthomycin A: Asymmetric Total Synthesis and Structural Determination,
Kuanwei Chen, Tao Xie, Yanfang Shen, Haibing He, Xiaoli Zhao, Shuanhu Gao,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c00193

![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)

