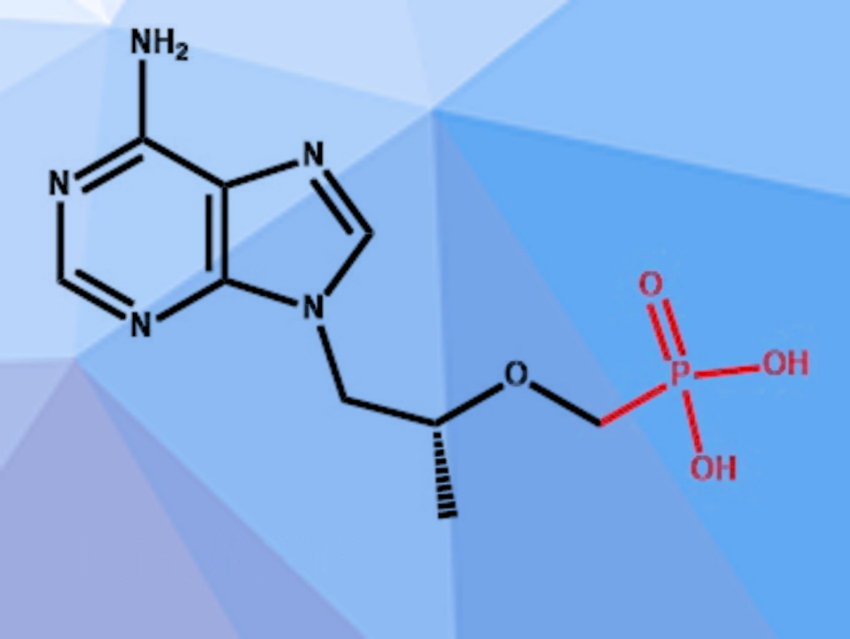
Tenofovir (pictured) is a nucleotide reverse transcriptase inhibitor that is commonly used to treat HIV and hepatitis B. It has also shown promising activity against SARS-CoV-2 in vitro and there is some evidence that fewer severe COVID-19 cases and fatalities occur in HIV patients receiving a treatment regime that includes tenofovir. The established synthesis of tenofovir uses an ethyl phosphonate intermediate, which can be synthesized from cheap and commercially available diethyl phosphite, but requires expensive reagents and elaborate workup procedures for the last synthesis step.
Till Opatz, University of Mainz, Germany, and colleagues have developed an alternative route, using di-tert-butyl phosphite instead of diethyl phosphite as the phosphonic acid precursor. The team synthesized di-tert-butyl phosphite from phosphorus trichloride and sodium tert-butoxide in 2-methyl-tetrahydrofuran. The compound was then hydroxymethylated using paraformaldehyde in acetonitrile and a mesylate group was installed. The resulting intermediate was reacted with (R)-9-(2-hydroxypropyl)adenine (HPA) and the tert-butyl groups were cleaved off to obtain tenofovir.
The tert-butyl-phosphite is more prone to hydrolyzation than the ethyl equivalent. This makes the synthesis of the starting material more challenging, but the same property leads to much easier hydrolysis of the phosphonate ester in the final step of the synthesis. The team obtained tenofovir in a yield of 72 % on a 5 g scale.
- Di-tert-butyl Phosphonate Route to the Antiviral Drug Tenofovir,
Jule-Philipp Dietz, Dorota Ferenc, Timothy F. Jamison, B. Frank Gupton, Till Opatz,
Org. Process Res. Dev. 2021.
https://doi.org/10.1021/acs.oprd.0c00473