Ketones can be synthesized via the hydroacylation of alkenes. These types of reactions can be catalyzed using transition metals. However, most intermolecular hydroacylation reactions have anti-Markovnikov selectivity, i.e., they form linear ketones and not branched ones. Markovnikov-selective hydroacylations usually require activated alkenes as substrates. A general method for the Markovnikov-selective hydroacylation of unactivated alkenes would, thus, be useful.
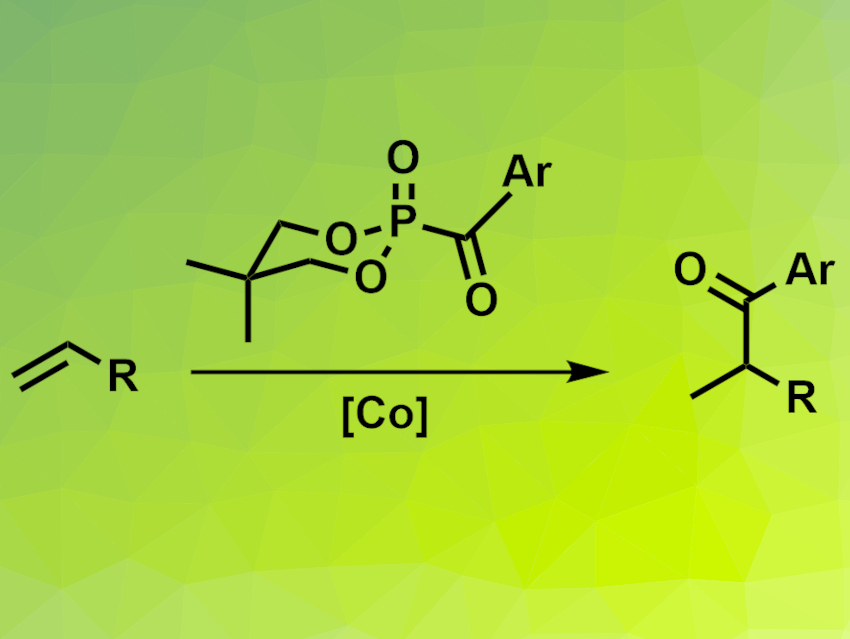
Yewen Fang, Ningbo University of Technology, China, Chaozhong Li, Ningbo University of Technology and Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and colleagues have developed a method for the Markovnikov-selective conversion of unactivated alkenes (pictured). The team used a cobalt salen complex as the catalyst and acylphosphonates with a 5,5-dimethyl-1,3,2-dioxophosphinanyl skeleton (pictured) as acylating agents. Phenylsilane served as a hydrogen source, tert-butyl hydroperoxide (TBHP) as the oxidant, and 1-chloromethyl-4-fluorodiazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (Selectfluor) as an additive.
The team obtained the desired ketones in moderate to good yields and with the targeted Markovnikov selectivity from a variety of terminal alkenes and several different acylphosphonates. They propose a mechanism that involves a Co(III) hydride intermediate, which undergoes a hydrogen atom transfer (HAT) reaction with the alkene to give an alkyl radical. This radical attacks the carbonyl group of the acylphosphonate to give the desired hydroacylation product.
- Cobalt-Catalyzed Markovnikov-Selective Radical Hydroacylation of Unactivated Alkenes with Acylphosphonates,
Benxiang Zhang, Jiayan He, Yi Li, Tao Song, Yewen Fang, Chaozhong Li,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c02629


![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
