Organic reactions are most often performed in liquid solvents. However, poorly soluble substrates, such as polyaromatic compounds, either require large amounts of solvent or cannot be reacted in solution at all. Such compounds are interesting, e.g., for optical or semiconductor applications—thus, more efficient ways to synthesize and functionalize them would be useful.
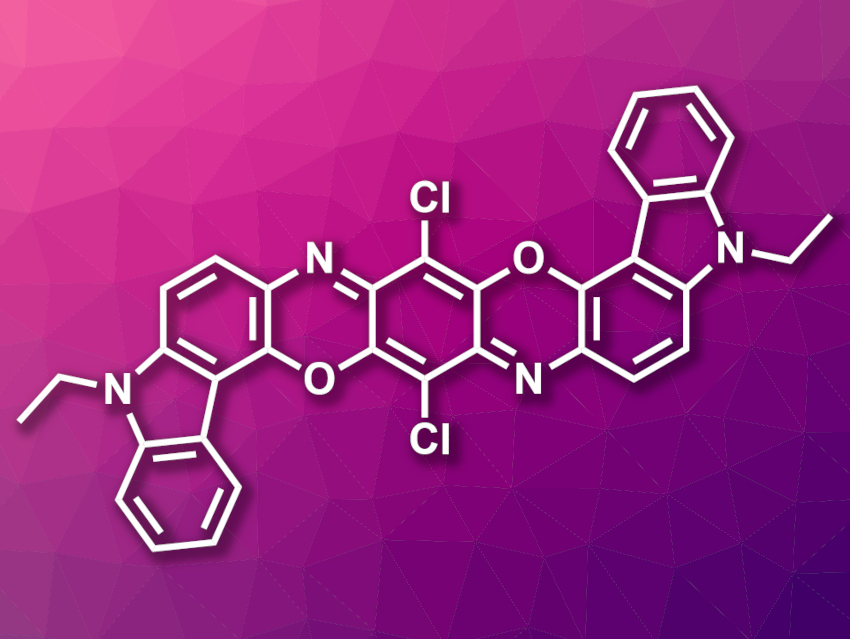
Koji Kubota, Hajime Ito, Hokkaido University, Sapporo, Japan, and colleagues have developed an efficient solid-state reaction protocol that can be used for cross-couplings of poorly soluble aryl halides with large polyaromatic structures, i.e., reactions that cannot be performed in solution. The method is based on palladium-catalyzed Suzuki–Miyaura cross-coupling reactions, which are performed under high temperatures using ball milling. The team coupled a variety of poorly soluble or insoluble aryl halides (example pictured) with arylboronic acids, using a catalytic system consisting of Pd(OAc)2, the phosphine ligand SPhos, and 1,5-cyclooctadiene (1,5-cod). The reactions were conducted in a stainless-steel ball mill with stainless-steel balls, which was heated using a heat gun set to 250 °C.
For poorly soluble substrates, the solid-state reactions were much faster and gave significantly higher yields than solution-based reactions. The method can even be used for aryl halides that can be classified as insoluble, which enables entirely new reactions.
- Tackling Solubility Issues in Organic Synthesis: Solid-State Cross-Coupling of Insoluble Aryl Halides,
Tamae Seo, Naoki Toyoshima, Koji Kubota, Hajime Ito,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c00906

![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)

