Pyridines are present in many biologically active molecules. Methods for the regioselective functionalization of pyridines are useful, e.g., for pharmaceutical chemistry. However, pyridine functionalization reactions often require multiple steps, have a narrow substrate scope, or provide only limited regioselectivity.
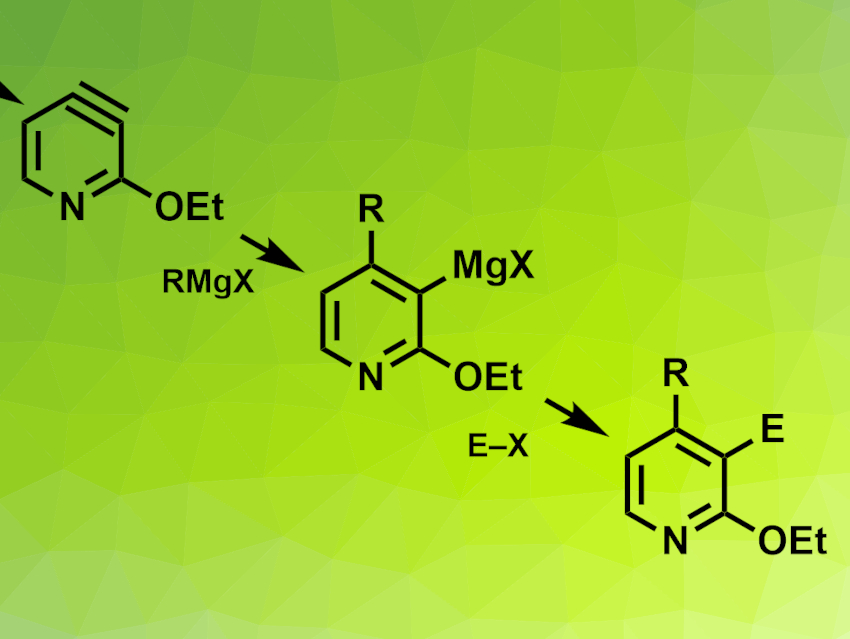
Paul Knochel, Ludwig Maximilian University of Munich, Germany, and colleagues have developed a regioselective 3,4-difunctionalization of 3-chloropyridines via 3,4-pyridyne intermediates. Pyridynes (example pictured top left) are reactive intermediates, similar to benzyne. The team started from 3-chloro-2-ethoxypyridine, which was lithiated using n-BuLi and transmetalated with an RMgBr·LiCl-type compound. An elimination reaction then gave a 3,4,-pyridyne intermediate, which was reacted with a Grignard reagent and an electrophile to give the desired difunctionalized products (pictured).
The products were obtained in moderate to good yields. To demonstrate the applicability of the method, the researchers used it to synthesize a key intermediate for (±)-paroxetine, an antidepressant drug. The reaction can also be used in a continuous flow set-up.
- Regioselective difunctionalization of pyridines via 3,4-pyridynes,
Benjamin Heinz, Dimitrije Djukanovic, Paolo Filipponi, Benjamin Martin, Konstantin Karaghiosoff, Paul Knochel,
Chem. Sci. 2021.
https://doi.org/10.1039/d1sc01208h
![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)


