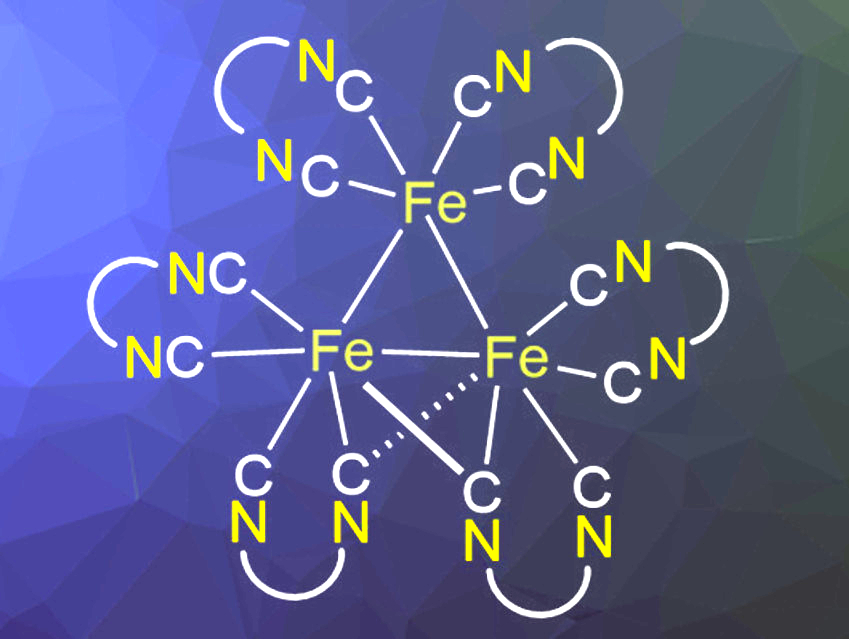
Isonitriles can coordinate metals in a manner similar to CO ligands. Chelating bis(isonitriles) as ligands increase the rigidity of complexes and can, e.g., be used in photocatalytically active complexes of transition metals like copper or molybdenum. The use of cheap and abundant iron for this purpose would be highly desirable, but so far, the excited-state lifetimes of iron complexes are too short for photocatalytic applications. Bidentate isonitrile ligands could potentially form iron complexes with more suitable photochemical properties. However, there has been only limited data on such iron complexes.
Robert Wolf, Oliver Reiser, University of Regensburg, Germany, and colleagues have synthesized a series of iron complexes of the chelating bis(isonitrile) ligands bis(2-isocyanophenyl)phenylphosphonate (BINC) and the bulkier 2,2″-diisocyano-3,5,3″,5”tetramethyl-1,1′:3′,1″-terphenyl (CNAr3NC). The team first prepared a [FeBr2(BINC)2] precursor complex from iron bromide and BINC and then reduced it using KC8 to form the trinuclear complex [Fe3(BINC)6] (simplified structure pictured). The complex [Cp*Fe(BINC)]2 was synthesized via the KC8 reduction of the chloride-containing precursor complex [Cp*FeCl(BINC)]. Iron complexes of the bulkier isonitrile ligand CNAr3NC were synthesized using the same approach. The Fe(II) precursor complex with this ligand, [FeBr2(CNAr3NC)2], is analogous to the corresponding BINC complex. However, after reduction with KC8, a dimeric complex of the type [Fe(CNAr3NC)2]2 was formed.
The complexes were analyzed using single-crystal X-ray diffraction, NMR and infrared (IR) spectroscopy, and UV-Vis-NIR spectroelectrochemistry. [Fe(CNAr3NC)2]2 has a similar structure to the unstable iron carbonyl complex [Fe2(CO)8]. The CNAr3NC-based complex is stabilized by the bulky isonitrile ligands and can be isolated and fully characterized. According to the researchers, all synthesized complexes have structures similar to their CO counterparts. This shows that bidentate isonitrile ligands can replace two CO ligands with only minimal structural changes. The stabilizing ligands could also be useful for applications in catalysis.
- Synthesis and Characterization of Bidentate Isonitrile Iron Complexes,
Marion Till, John A. Kelly, Christoph G. P. Ziegler, Robert Wolf, Tianao Guo, Mark R. Ringenberg, Eugen Lutsker, Oliver Reiser,
Organometallics 2021.
https://doi.org/10.1021/acs.organomet.1c00042