Group 13 elements (M) can form a range of different compounds with nitrogen, e.g., amine complexes (R3MNR3), amides (R2MNR2), imides (RMNR), and nitrides (MN). Group 13 metal imides are usually not isolable as monomers because their di- or oligomerization is highly exothermic. Monomeric RAlNR-type compounds, for example, are challenging to obtain and require bulky substituents to prevent oligomerization. In particular, no compounds of this type in which Al and N are two-coordinate had been isolated and characterized so far.
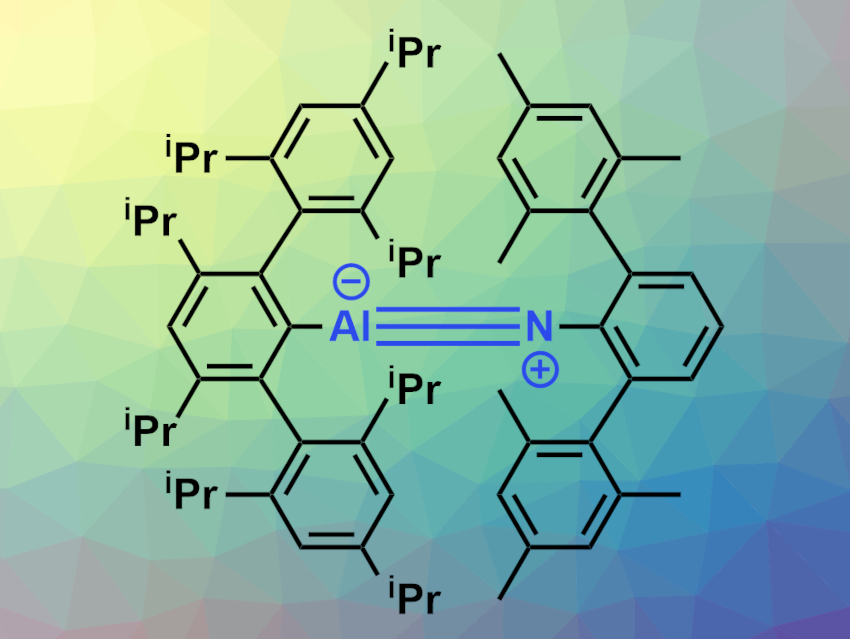
Heikki M. Tuononen, University of Jyväskylä, Finland, Philip P. Power, University of California, Davis, USA, and colleagues have prepared the monomeric aluminum imide AriPr8AlNArMe6 (pictured). The compound was synthesized via a reaction of the alanediyl :AlAriPr8 with the azide ArMe6N3 in hexanes. The reaction proceeds under loss of N2 within minutes at ambient temperature. The compound was characterized using X-ray crystallography, NMR, IR, and UV-Vis spectroscopy, and its electronic structure was investigated using density functional theory (DFT) calculations.
The team found that the compound has short Al–N distances of around 1.63 Å with linear C–Al–N–C substructures. These short bond distances and linear structure provide evidence for an Al≡N triple-bond character. This was supported by the results of the DFT calculations.
- A Monomeric Aluminum Imide (Iminoalane) with Al–N Triple-Bonding: Bonding Analysis and Dispersion Energy Stabilization,
Joshua D. Queen, Sini Irvankoski, James C. Fettinger, Heikki M. Tuononen, Philip P. Power,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c02463