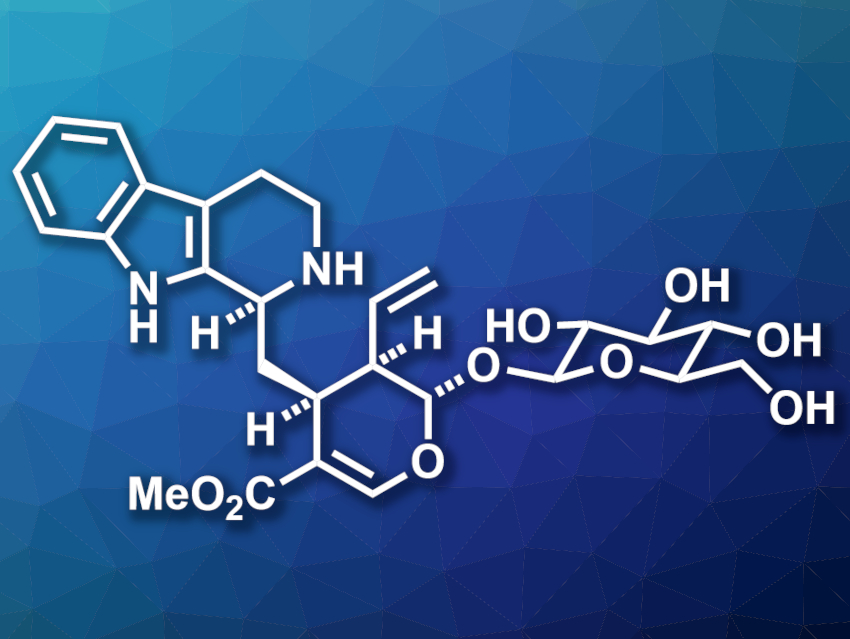
Monoterpene indole alkaloids are an important class of natural products with many bioactive examples, e.g., quinine and strychnine. (−)-Strictosidine (pictured) is a common biosynthetic precursor to these natural products and could be a useful intermediate for the synthesis of derivatives. However, it is challenging to access by organic synthesis.
Yi Tang, K. N. Houk, Neil K. Garg, University of California, Los Angeles, USA, and colleagues have synthesized (−)-strictosidine using a combination of organic synthesis and biocatalysis. The team first used a Diels–Alder reaction between a glucosyl-functionalized alkene and an enal to access the dihydropyran unit of the target compound. Then they converted the resulting intermediate to (−)-secologanin by introducing the terminal olefin group and creating an aldehyde functionality. Finally, they used an enzymatic Pictet–Spengler reaction between (−)-secologanin and tryptamine, catalyzed by the enzyme strictosidine synthase, to introduce the indole substructure and obtain (−)-strictosidine.
Overall, (−)-strictosidine was obtained in ten steps. The researchers also synthesized new, unnatural analogues of strictosidine. Using a similar overall synthetic approach, they prepared epi-strictosidine isomers that are difficult to access otherwise. They also modified the tryptamine building block to create a precursor for the formation of a reactive “tryptaminyne”, i.e., a benzyne analogue. As a result, the team obtained “strictosidyne” and “strictosamidyne” precursors that could be useful for further functionalization.
- Total Synthesis of (−)-Strictosidine and Interception of Aryne Natural Product Derivatives “Strictosidyne” and “Strictosamidyne“,
Sarah M. Anthony, Veronica Tona, Yike Zou, Lucas A. Morrill, John M. Billingsley, Megan Lim, Yi Tang, K. N. Houk, Neil K. Garg,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c02004
![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)


