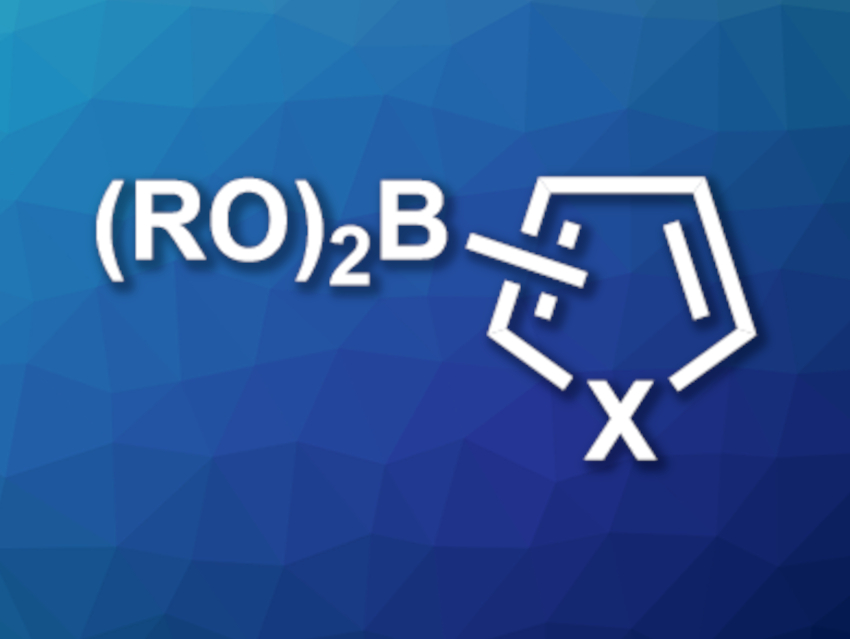
Organoboranes are useful intermediates in synthetic organic chemistry. Many borylation reactions rely on expensive metals such as iridium as catalysts. Alternative reactions using, e.g., cobalt or iron are hampered by issues such as high toxicity or limited substrate scopes.
Michael J. Ingleson, University of Edinburgh, UK, and colleagues have developed an electrophilic C–H borylation of heteroarenes (example product pictured, X = N,O,S) that is catalyzed by inexpensive and relatively non-toxic zinc. The team used cationic zinc complexes of the type [IDippZnEt][B(C6F5)4] (IDipp = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) together with N,N-dimethyl-p-toluidine (DMT) as the catalyst system, pinacol borane (HBPin) or catechol borane (HBCat) as borylation reagents, and chlorobenzene as the solvent.
The team borylated, e.g., activated indoles and 2-methylthiophene using pinacolborane. With catecholborane, the researchers observed an improved substrate scope, including less activated indoles, 2-methylfuran, and unsubstituted thiophene. They propose a mechanism that involves the formation of a borenium-type intermediate from the hydroborane, which then leads to the desired C–H borylation.
- Zinc catalysed electrophilic C–H borylation of heteroarenes,
Matthew E. Grundy, Kang Yuan, Gary S. Nichol, Michael J. Ingleson,
Chem. Sci. 2021.
https://doi.org/10.1039/d1sc01883c