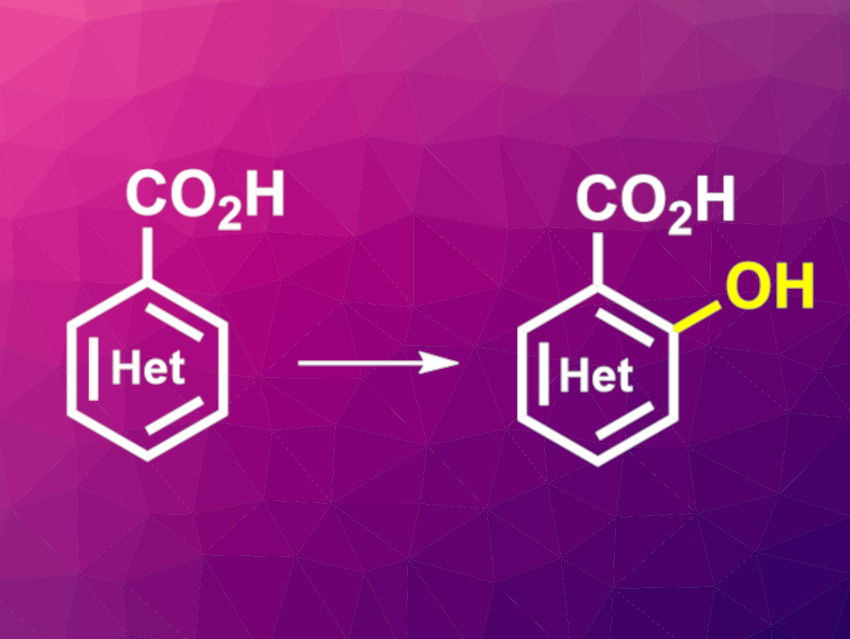
Hydroxylation reactions of aromatic compounds are important in organic synthesis. Such hydroxylations of aryl C–H bonds can be performed using transition-metal catalysts. However, this is difficult when molecular oxygen is used as the oxidant.
Jin-Quan Yu, The Scripps Research Institute, La Jolla, CA, USA, and colleagues have developed a protocol for the directed C‒H hydroxylation of both aromatic and heteroaromatic carboxylic acids with molecular oxygen. The team used a palladium complex with a bidentate pyridine-pyridone ligand that efficiently catalyzes this reaction at positions adjacent to carboxylic acids. They converted a variety of aromatic and heterocyclic carboxylic acids using Pd(OAc)2 as the catalyst, 6-(1-cyclohexyl-1-(5-methylpyridin-2-yl)ethyl)pyridin-2(1H)-one as the ligand, 1,4-benzoquinone as an additive, dimethylformamide (DMF) as the solvent, and O2 as the oxidant.
The desired hydroxylated products were obtained in mostly good yields. According to the researchers, the ligand binds to the Pd through the pyridine and pyridone components, and a tautomerization reaction involving the ligand leads to two different coordination modes that play a role in the catalytic cycle. The reaction allows the late-stage hydroxylation of complex molecules at sites that were previously difficult to access.
- A tautomeric ligand enables directed C‒H hydroxylation with molecular oxygen,
Zhen Li, Zhen Wang, Nikita Chekshin, Shaoqun Qian, Jennifer X. Qiao, Peter T. Cheng, Kap-Sun Yeung, William R. Ewing, Jin-Quan Yu,
Science 2021.
https://doi.org/10.1126/science.abg2362