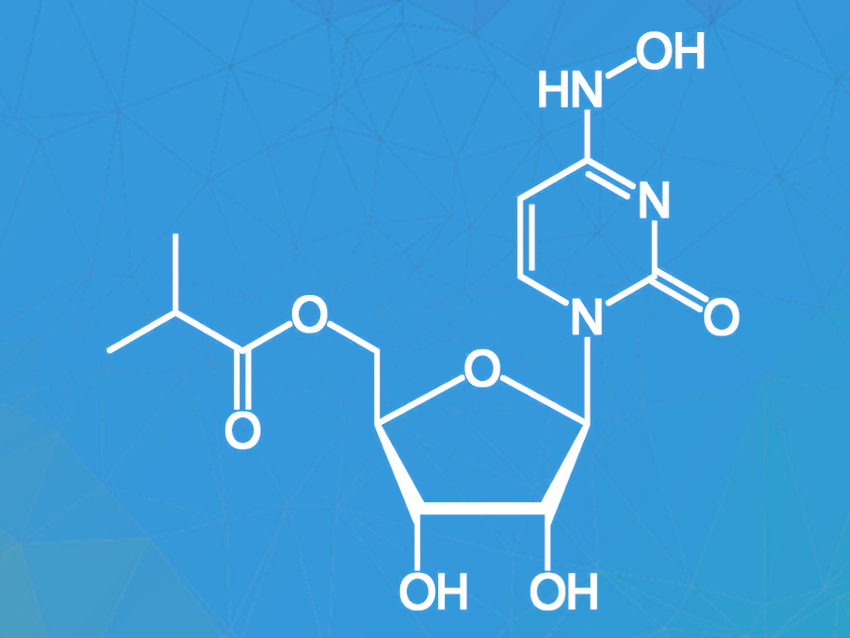
Molnupiravir (pictured) is an antiviral drug candidate undergoing clinical trials as a potential treatment for COVID-19. Studies in mice have shown that administration of molnupiravir leads to lower virus titers and suppresses virus transmission. Easy oral applicability and a relatively simple chemical structure allowing for a fast synthesis of molnupiravir are additional benefits.
Molnupiravir can be synthesized from the commercially available nucleoside cytidine in only two steps. This starting material is cheap, abundant, and frequently used in food additives and animal feed. The available synthesis routes are not ideal, however, because they require chromatographic purification, large amounts of solvents, and a high enzyme loading for the enzymatic esterification of the intermediate.
David R. Snead, Medicines For All Institute, Richmond, VA, USA, and colleagues have developed an improved two-step synthesis of molnupiravir from cytidine with an overall yield of 60 %. The first step in the synthesis of molnupiravir from cytidine is the hydroxamination of cytidine with hydroxylamine to give N-hydroxycytidine (NHC). The original yield of the reaction was only 50 % and the reaction required large amounts of solvent and chromatographic purification. Substituting hydroxylamine with hydroxylamine sulfate and using pure water instead of an isopropanol-water mixture solved these problems. NHC was easily purified by crystallization as the hydrate in high purity.
In the second synthesis step, NHC is enzymatically acylated to give the target compound. The previous synthesis required a very high enzyme loading (200 wt%) and large quantities of the harmful solvent 1,4-dioxane, and the yield was reduced by the formation of an oxime-ester byproduct. These issues were tackled by using 2-methyl THF as a substitute for dioxane and running the reaction at higher concentrations with only 20 wt% enzyme loading. The byproduct was successfully converted to the target product by addition of hydroxylamine. Crystallization from water yielded molnupiravir in high purity and 71 % yield.
The overall yield was improved from 37 % to 60 % and both reaction steps were successfully upscaled to a 0.5 kg scale. The synthesis route does not require chromatographic purification and uses environmentally benign solvents and cheap starting materials. Therefore, it could be suitable for the low-cost, large-scale production of molnupiravir.
- Toward a Practical, Two-Step Process for Molnupiravir: Direct Hydroxamination of Cytidine Followed by Selective Esterification,
Dinesh J. Paymode, N. Vasudevan, Saeed Ahmad, Appasaheb L. Kadam, Flavio S.P. Cardoso, Justina M. Burns, Daniel W. Cook, Rodger W. Stringham, David R. Snead,
Org. Process Res. Dev. 2021.
https://doi.org/10.1021/acs.oprd.1c00033
Also of Interest
News: Coronavirus Drug Molnupiravir
- 05 October 2021
Molnupiravir significantly reduces risk of hospitalization and/or death, according to Merck
News: Improved Synthesis Route for Molnupiravir
- 04 August 2021
Efficient synthesis from cheap and easily available cytidine
Research Highlight: How Molnupiravir Causes SARS-CoV-2 to Mutate and Die
Mechanism might apply to various viral polymerases and could explain broad-spectrum antiviral activity of molnupiravir
News: High-Yielding Synthesis of Antiviral Drug Candidate
- 19 November 2020
Improved preparation of EIDD‐2801, currently in clinical trials for COVID-19 treatment
News: Oral Drug Blocks SARS-CoV-2 Transmission in Ferrets
- 09 December 2020
Repurposed ribonucleoside analogue inhibitor of influenza viruses