Oxyfluoride or oxide-fluoride is an important branch of mixed anion compounds. In recent years, oxyfluorides with various types of fluorinated [MOm–nFn] (M = B, P, S, Si; m = 4, 6; n = 1–3, etc.) units have been designed, and solid-state chemistry has been extended to include systems such as fluoroxoborates, fluorophosphates, fluoroxosilicates, and fluoroxosulfates. Borates and phosphates can be combined to form compounds with B-O and P-O bonds: (i) borophosphates (B-O and P-O units are linked) and (ii) borate phosphates (both anion groups are isolated). The number of borate phosphates is very limited compared to borophosphates.
Miriding Mutailipu and Shilie Pan, Xinjiang Key Laboratory of Electronic Information Materials and Devices, Urumqi, China, and University of Chinese Academy of Sciences, Beijing, China, and colleagues have synthesized the first inorganic oxyfluoride with both B–F and P–F bonds by combining fluoroborates and fluorophosphates. The International Union of Pure and Applied Chemistry (IUPAC) name for (NH4)3[PO3F][BF4] should be ammonium tetrafluoroborate-monofluorophosphate.
The team grew single crystals of (NH4)3[PO3F][BF4] via liquid phase neutralization at room temperature. A mixture of Na2PO3F, (NH4)HF2, and H3BO3 with the molar ratio of 1:3:1 was dissolved in 50 mL deionized water and stirred until it was completely clear. After several days, colorless (NH4)3[PO3F][BF4] crystals were obtained at the bottom of the beaker in about 70 % yield.
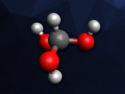
(NH4)3[PO3F][BF4] crystallizes in the monoclinic crystal system with the space group P21/m. The structure of (NH4)3[PO3F][BF4] consists of three isolated units: [NH4], [PO3F], and [BF4]. In an asymmetric unit, there are three crystallographically independent [NH4] ammonium cations, one [PO3F] monofluorophosphate anion, and one [BF4] perfluorinated anion. The researchers found that all [PO3F] and [BF4] units are in an isolated configuration without any linkage, so (NH4)3[PO3F][BF4] can be classified as fluoroborate-fluorophosphates.
The researchers believe that their work is of great importance in enriching the solid-state chemistry of borates and phosphates and opens a new branch of mixed anion compounds with fluoroborate-fluorophosphates.
- Tetrafluoroborate-Monofluorophosphate (NH4)3[PO3F][BF4]: First Member of Oxyfluoride with B–F and P–F Bonds,
Haotian Qiu, Wenbing Cai, Zhihua Yang, Yanli Liu, Miriding Mutailipu, Shilie Pan,
ACS Org. Inorg. Au 2021.
https://doi.org/10.1021/acsorginorgau.1c00018
![Novel Oxyfluoride (NH4)3[PO3F][BF4]](https://www.chemistryviews.org/wp-content/uploads/legacy/common/images/thumbnails/source/17b3a283392.png)