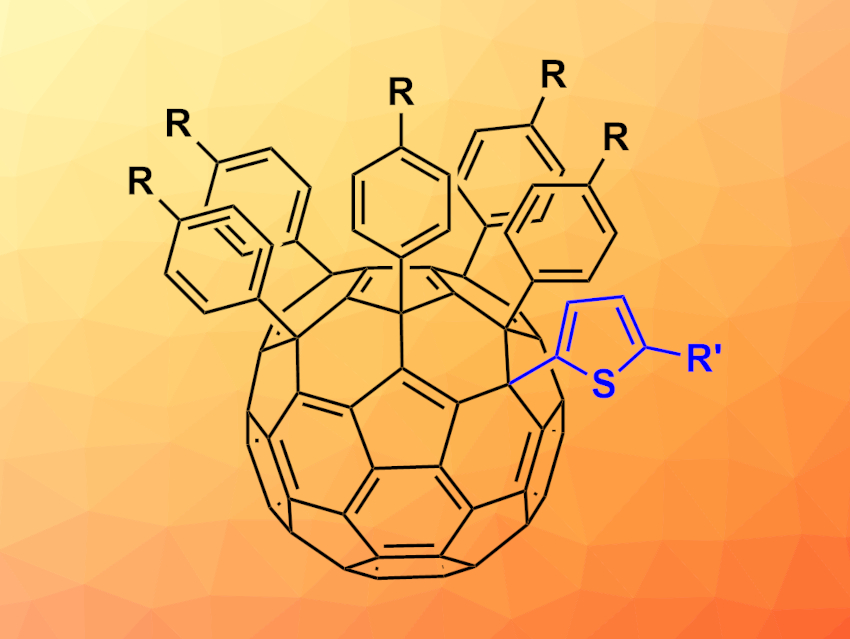
Fullerenes, such as C60, can be used, e.g., as a basis for functional materials or pharmaceutically active compounds. However, the functionalization of fullerenes can lead to a large number of isomers when multiple functional groups are involved. Selective approaches for the introduction of fullerene substituents are, thus, useful.
Olga A. Kraevaya, Institute for Problems of Chemical Physics of the Russian Academy of Sciences (RAS), Chernogolovka, Russia, and colleagues have developed a reaction that converts fullerene derivatives of the type C60Ar5Cl to fullerenes with thiophene residues of the type C60Ar5(thiophene) (pictured). The team started from a set of C60Ar5Cl precursors (Ar = C6H5 or p-C6H4(CH2)nCOOMe with n = 1,2,3). These precursors were reacted with a variety of substituted thiophenes in the presence of SnCl4 and water in 1,2-dichlorobenzene at 80 °C.
The desired thiophene-functionalized products were obtained in yields of 40–85 %. The ester functionalities in the products can be hydrolyzed to give water-soluble fullerene-based polycarboxylic acids. These compounds were evaluated for their biological activities. The researchers found promising antiviral activities against human immunodeficiency viruses (HIV) and influenza viruses.
- Water-Promoted Reaction of C60Ar5Cl Compounds with Thiophenes Delivers a Family of Multifunctional Fullerene Derivatives with Selective Antiviral Properties,
Olga A. Kraevaya, Valeriya S. Bolshakova, Alexander S. Peregudov, Alexander V. Chernyak, Nikita A. Slesarenko, Vitaliy Yu. Markov, Natalia S. Lukonina, Vyacheslav M. Martynenko, Ekaterina O. Sinegubova, Alexander F. Shestakov, Vladimir V. Zarubaev, Dominique Schols, Pavel A. Troshin,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c02623