Substituted indeno[1,2-b]indoles (pictured above) have interesting biological and electronic properties and could have applications, e.g., in solar cells. Previous work shows that the related indolo[1,2-b]indoles can be created from 2-alkynylaniline through bisamination with di-tert-butyl-aziridinone via an indole-fused pallada(II)cycle [1]. This pallada(II)cycle could be a useful intermediate in the synthesis of indeno[1,2-b]indoles.
Yian Shi, Changzhou University, China, Colorado State University, Fort Collins, USA, and colleagues have created an efficient palladium-catalyzed reaction sequence based on a nucleophilic aminopalladation and a carbene insertion that can be used to synthesize indeno[1,2-b]indoles. The indeno[1,2-b]indoles were prepared from various bromide-substituted 2-alkynylaniline derivatives via a reaction with an α-diazo ester in the presence of Pd(TFA)2 (TFA = trifluoroacetate) as a catalyst, 1,1′-bis(dicyclohexylphosphino)ferrocene as a ligand, and K2CO3 as a base in CH3CN as the solvent. The reaction was performed at 90 °C.
Using this protocol, the team obtained a variety of substituted indeno[1,2-b]indoles in yields of 50–99 %. Compared with other synthesis options such as the Fischer indole synthesis, enamine formation, and metal-catalyzed cyclizations, this approach could provide access to a broader variety of structures. The reaction can also be used to synthesize conjugated bisindeno[1,2-b]indoles (example product pictured below).
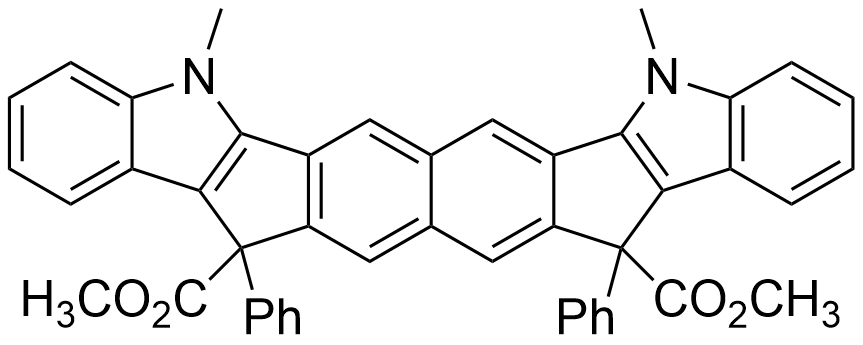
- A Tandem Nucleophilic Aminopalladation and Carbene Insertion Sequence for Indole Fused Polycycles,
Sudarshan Debnath, Mei Lu, Lingli Liang, Yian Shi,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c02512
Reference
- [1] Domino C–N Bond Formation via a Palladacycle with Diaziridinone. An Approach to Indolo[3,2-b]indoles,
Sudarshan Debnath, Lingli Liang, Mei Lu, Yian Shi,
Org. Lett. 2021, 23, 3237–3242.
https://doi.org/10.1021/acs.orglett.1c00466

![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)

