Transition-metal catalyzed cross-coupling reactions are among the most important synthetic transformations in organic chemistry. Many of these reactions are catalyzed by palladium complexes, but due to the high cost of these catalysts, the use of more abundant metals is desirable. One of these more abundant metals suitable for some cross-coupling reactions is copper.
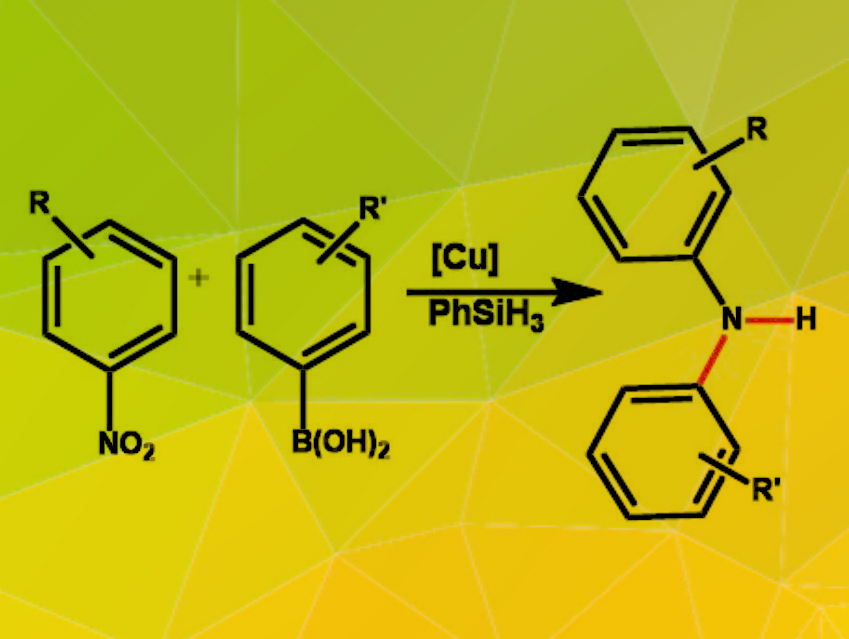
Tom G. Driver, University of Illinois at Chicago, USA, and colleagues have developed a copper-catalyzed cross-coupling reaction for the synthesis of a wide range of diarylamines (pictured). The team coupled aryl boronic acids with easily synthesizable nitroarenes. Phenyl silane was used as a stoichiometric reductant and copper(II)acetate, combined with the diphosphine ligand 1,4-bis(diphenylphosphino)butane (dppb), as a catalyst. The reactions were carried out in an acetonitrile/toluene mixture at 60 °C.
The reaction tolerates a broad range of functional groups on both the nitroarene and the aryl boronic acid. For para-substituted nitroarenes, electron-poor substrates gave the best results, while nitroarenes with electron-donating groups did not undergo the reaction. In meta– or ortho-position, a wide range of different functional groups were tolerated. For the boronic acid, different types of substituents were tolerated in the meta– and para-positions. According to the researchers, mechanistic investigations indicate that the reaction proceeds via a nitrosoarene intermediate. The protocol enables access to a wide range of differently functionalized diarylamines under comparatively mild conditions from easily available starting materials.
- Cu-Catalyzed Cross-Coupling of Nitroarenes with Aryl Boronic Acids to Construct Diarylamines,
Xinyu Guan, Haoran Zhu, Tom G. Driver,
ACS Catalysis 2021.
https://doi.org/10.1021/acscatal.1c03113