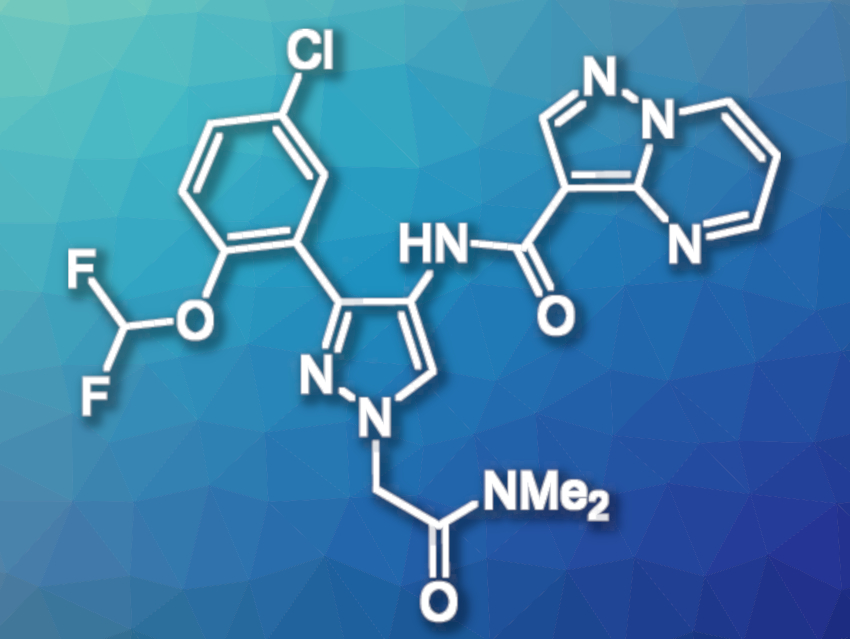
GDC-4379 (pictured) is an inhibitor of the enzyme Janus kinase 1 (JAK1), which regulates signaling pathways involving type-2-cytokines. These cytokines may cause unwanted inflammation, which contributes to asthma attacks. The inhibition of JAK1 by GDC-4379 could be useful to treat patients with severe asthma. The compound is administered by a dry powder inhaler. This requires crystallization methods that provide a material that has appropriate surface properties for use in inhalers and that allows micronization to achieve particle diameters smaller than 5 μm.
Andreas Stumpf, Genentech Inc., San Francisco, CA, USA, and colleagues have developed an efficient synthesis route that provides multiple kilograms of GCD-4379 in the appropriate crystalline structure. The target molecule was prepared from two fragments that were synthesized separately. First, pyrazolo[1,5-a]-pyrimidine-3-carboxylic acid was prepared from commercially available ethyl-2-cyanoacetate, which was subjected to a Knoevenagel condensation with trimethyl orthoformate. The condensation product was then transformed to the target intermediate by a reaction with hydrazine hydrate, directly followed by condensation with malonaldehyde bis(dimethylacetal) and hydrolysis of the resulting ester.
The second key intermediate, 3-(5-chloro-2-(difluoromethoxy)phenyl)-1H-pyrazol-4-amine, was synthesized from 5-chloro-2-hydroxyacetophenone, which was transformed to a difluoromethoxy ether by alkylation with chlorodilfluoromethane. The crude ether was subjected to a Claisen condensation with ethyl formate, followed by oximation and condensation with hydrazine. The resulting intermediate was then converted to the corresponding amine by a CuCl-catalyzed sodium borohydride reduction.
The two key intermediates were then transformed to the target product by a Schotten-Baumann amidation, followed by alkylation with 2-bromo-N,N-dimethylacetamide. The crude GDC-4379 was purified by crystallization. The crude product was first crystallized from a mixture of dimethyl sulfoxide (DMSO) and methanol, followed by a second crystallization step from a mixture of ethyl L-lactate and water. The team obtained pure target product in the hydrate form and crystal structure needed for the intended use in inhalers.
- Efficient, Protecting Group Free Kilogram-Scale Synthesis of the JAK1 Inhibitor GDC-4379,
Andreas Stumpf, Johannes Burkhard, Di Xu, Andreas Marx, David Lao, Miriam Ochsenbein, Rohit Ranjan, Remy Angelaud, Francis Gosselin,
Org. Process Res. Dev. 2021.
https://doi.org/10.1021/acs.oprd.1c00302