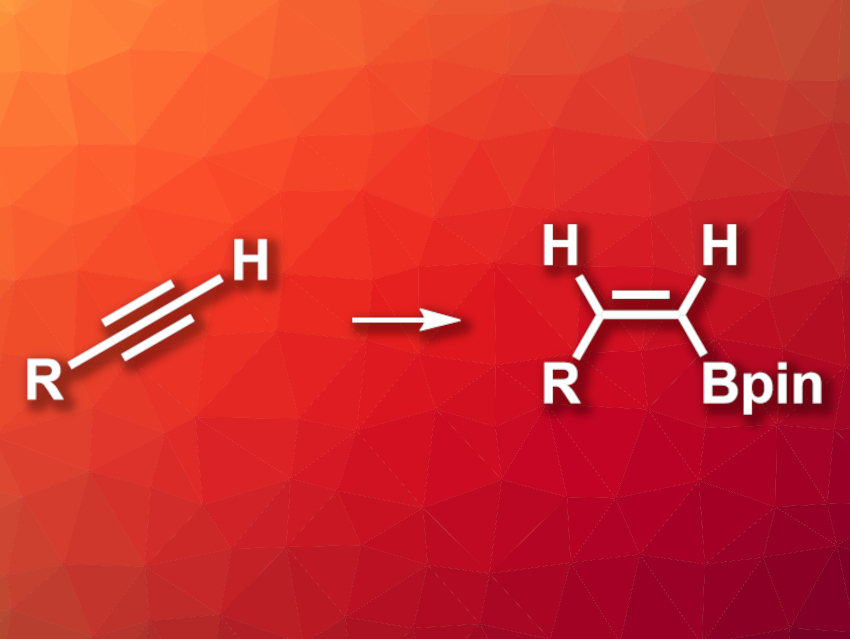
Hydroborations are very commonly used reactions in organic synthesis. For example, alkenyl boronic esters can be synthesized via the hydroboration of alkynes and serve as useful intermediates. There are a variety of examples of transition-metal-catalyzed hydroboration of alkynes to give (E)-alkenyl boronates. (Z)-selective hydroboration reactions of alkynes are less common.
Nobuharu Iwasawa, Tokyo Institute of Technology, Japan, and colleagues have developed a (Z)-selective hydroboration of terminal alkynes (pictured). The team used a rhodium catalyst with a thioxanthene-based PSP-type pincer ligand. They reacted 13 different terminal alkenes with pinacolborane (HBpin) in the presence of [Rh(nbd)2]BF4 (nbd = norbornadiene) and the pincer ligand, using toluene as the solvent. The reactions were performed at 30 °C.
The desired (Z)-alkenyl boronates were obtained in moderate to good yields and with high selectivities. The reaction tolerates a range of functional groups, including ketones and halides. The team proposes a mechanism that involves Rh–vinylidene intermediates.
- (Z)-Selective Hydroboration of Terminal Alkynes Catalyzed by a PSP–Pincer Rhodium Complex,
Yanzong Lyu, Naoyuki Toriumi, Nobuharu Iwasawa,
Org. Lett. 2021.
https://doi.org/10.1021/acs.orglett.1c03606
![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)


