Heavier analogues of carbenes and their dimers, which can be considered heavier analogues of alkenes, are interesting research targets. However, such dimers can be challenging to synthesize due to their lower bond strengths than those in alkenes. Distannenes (R2Sn–SnR2), for example, typically dissociate into stannylenes (R2Sn:) in solution. To compensate for weak Sn–Sn bonds, bulky substituents and the London dispersion forces between them can be employed to stabilize such dimers.
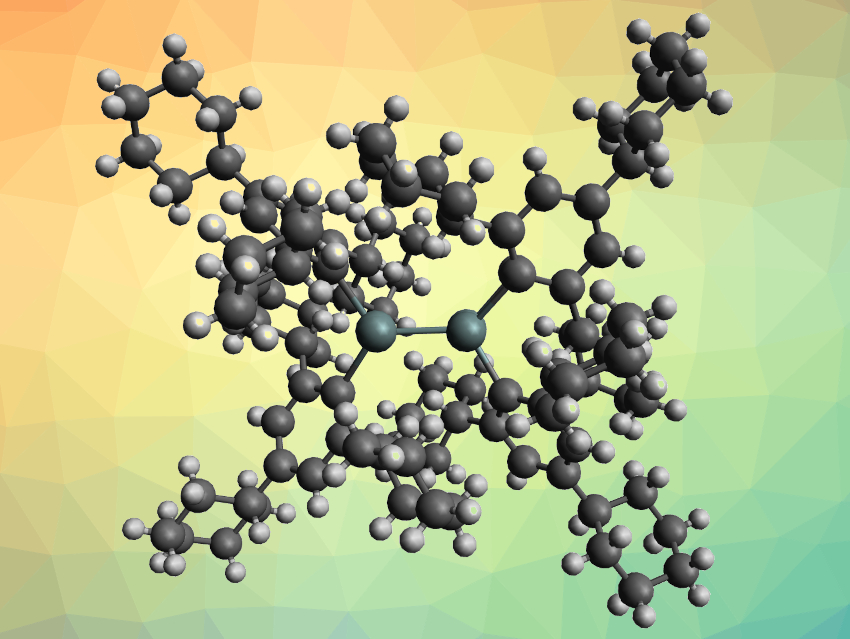
Stefan Grimme, University of Bonn, Germany, Philip P. Power, University of California, Davis, USA, and colleagues have synthesized a solution-stable distannene, {Sn(C6H2-2,4,6-Cy3)2}2 (pictured, Cy = cyclohexyl). The compound was prepared via the reaction of a lithium salt of the bulky ligand, {Li(C6H2-2,4,6-Cy3)·OEt2}2, with SnCl2 in diethyl ether and obtained as a red, crystalline product.
The trans-pyramidalized structure of the resulting distannene, which was determined by X-ray crystallography, features a short Sn–Sn distance of ca. 2.70 Å, in spite of the bulky ligands. There are several close contacts between the cyclohexyl rings of the two ligands, which indicates London dispersion attraction. The team found that the distannene remains dimeric in solution, even at elevated temperatures. Density functional theory (DFT) calculations confirmed that this stability is due to London dispersion attraction between the two R2Sn groups—when dispersion effects were excluded, the monomer was favored. When the team used a less bulky 2,4,6-triphenylphenyl ligand, they found that the product is monomeric, which provides additional evidence for the importance of the dispersion forces between the two bulky ligands.
- Designing a Solution-Stable Distannene: The Decisive Role of London Dispersion Effects in the Structure and Properties of {Sn(C6H2-2,4,6-Cy3)2}2 (Cy = Cyclohexyl),
Cary R. Stennett, Markus Bursch, James C. Fettinger, Stefan Grimme, Philip P. Power,
J. Am. Chem. Soc. 2021.
https://doi.org/10.1021/jacs.1c09976