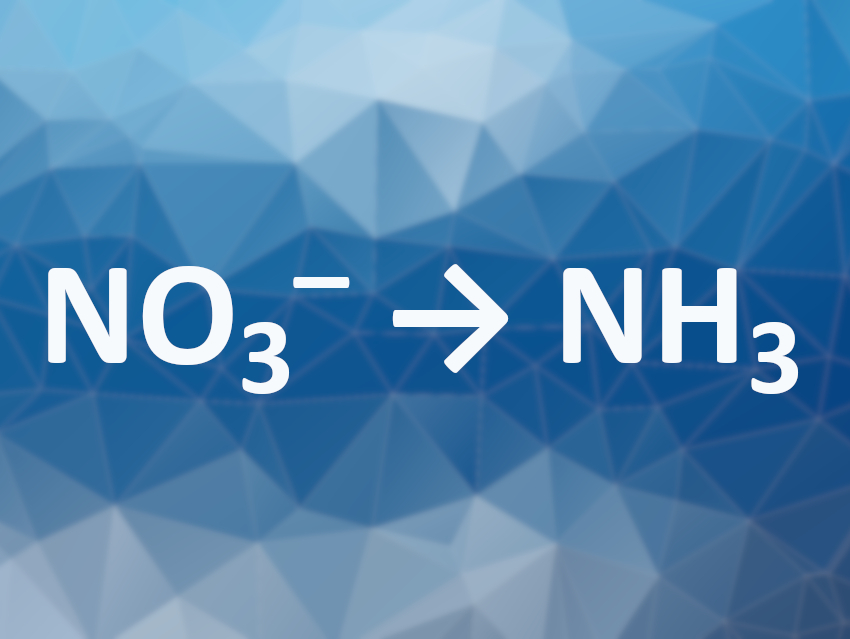
Ammonia is usually synthesized from N2 via the Haber–Bosch process. However, this process needs large amounts of energy. Using nitrate as a substrate could be a useful alternative. Nitrate is often found in high concentrations in polluted water, e.g., due to the overuse of fertilizer. The electrochemical reduction of nitrate to ammonia could, thus, be particularly useful. However, the reaction involves a challenging eight-electron transfer process and competes with the hydrogen evolution reaction (HER). The development of catalysts that can selectively promote ammonia production from nitrate is, thus, an interesting research target.
Meng Gu, Southern University of Science and Technology, Shenzhen, China, Minhua Shao, The Hong Kong University of Science and Technology, Hong Kong SAR, China, and colleagues have prepared CoOx nanosheets enriched with surface oxygen to be used as a catalyst for the electrochemical conversion of nitrate to ammonia. The team synthesized the nanosheets by adding NaBH4 to a cobalt nitrate solution in the presence of cetyltrimethylammonium bromide (CTAB).
The researchers found that the nanosheets show good catalytic performance for the electrochemical nitrate reduction reaction. They achieve high ammonia yields and Faradaic efficiencies. The team performed density functional theory (DFT) calculations, which indicate that the adsorbed oxygen plays a critical role in suppressing the HER and boosting nitrate reduction. When the CoOx nanosheets were annealed to remove adsorbed oxygen, the ammonia yield was reduced and the HER activity was improved.
- Electrocatalytic Reduction of Nitrate to Ammonia on Low-Cost Ultrathin CoOx Nanosheets,
Jing Wang, Chao Cai, Yian Wang, Xuming Yang, Duojie Wu, Yuanmin Zhu, Menghao Li, Meng Gu, Minhua Shao,
ACS Catal. 2021.
https://doi.org/10.1021/acscatal.1c03918



Pt or Pd nanosheet can transform nitrate ion in hidroxilamin sulfate or chlorhidrate with very good catalitic performance. Hidroxilamin sulfate can transform cyclohexanon in cyclohexanon oxime and with Beckman reaction to E-caprolactam.