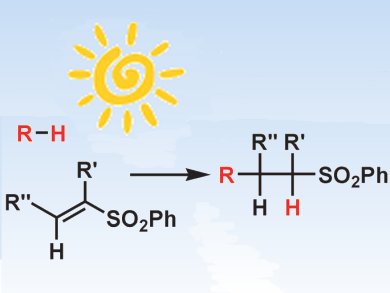
The sulfone moiety plays an important role in the synthesis of organic molecules due to the easy functionalization and the leaving group aptitude. Vinyl sulfones are suitable starting materials for the synthesis of substituted sulfones via carbon–carbon bond formation, as Michael acceptors in 1,4-addition reactions, as dienophiles in cycloadditions, as well as in conjugate nucleophilic radical addition reactions. The last method is appealing, but requires the use of harmful tin derivatives as radical chain carriers.
Maurizio Fagnoni and colleagues, University of Pavia, Italy, report a tin-free procedure for the radical conjugate addition onto vinyl sulfones. They used tetrabutylammonium decatungstate (TBADT) as a photocatalyst for the activation of the carbon–hydrogen bond from suitable donors. This skipped any preliminary functionalization and resulted in an atom-economical route. The products are useful building blocks and were obtained in satisfactory yields both under artificial UV light and under sunlight, so demonstrating the “green” potential of the process.
- A Tin-Free, Radical Photocatalyzed Addition to Vinyl Sulfones
D. Ravelli, S. Montanaro, M. Zema, M. Fagnoni, A. Albinia,
Adv. Synth. Catal. 2011.
DOI: 10.1002/adsc.201100591