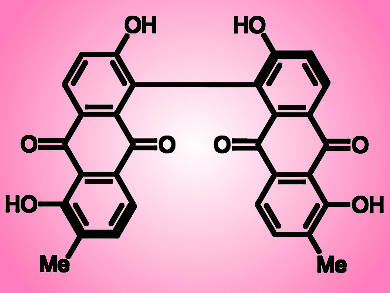
(S)-Bisoranjidiol is a photosensitizing chiral bisanthraquinone that generates electronically excited singlet oxygens when light shines on it. Because the compound is found in the Andean shrub Heterophyllaea pustulata (genera Rubiaceae), which grows in the mountains of Argentina and Bolivia, it represents a photo-toxic threat to grazing livestock. Understanding its chemistry isan important endeavor, not least because analogs of the compound may actually have medicinal activity that can be controlled by irradiation with light.
A team at the University of Pennsylvania, Philadelphia, USA, has used a neat sequence of reactions including an asymmetric oxidative biaryl coupling of a hindered 8-substituted 2-naphthol, selective para-quinone formation, and regioselective tandem Diels–Alder/aromatization reactions to construct the compound.
This approach represents an efficient entry into this natural product and also permits flexibility in the generation of further analogs to optimize biological properties.
- Enantioselective Total Synthesis of (S)-Bisoranjidiol, an Axially Chiral Bisanthraquinone,
E. E. Podlesny, M. C. Kozlowski,
Org. Lett. 2012.
DOI: 10.1021/ol3001365