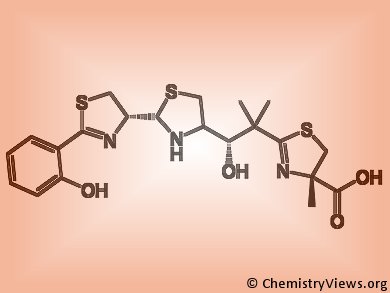
Microorganisms’ virulence depends on iron acquisition from host ferroproteins via siderophores, small, high affinity iron chelating molecules. Siderophores binding Zn(II) or Cu(II) have been also described, nevertheless their role in microorganisms’ pathogenicity in vivo is unknown.
Kaveri S. Chaturvedi and colleagues, Washington University School of Medicine, St. Louis, USA, clarified this issue by studying uropathogenic Escherichia coli strains. These bacteria produce multiple siderophores which include Fe(III) and Cu(II) binding catecholates as well as the phenolate yersiniabactin. During infections, yersiniabactin form complexes with host derived Cu(II) in order to prevent its binding to catecholate and its subsequent reduction to the bactericidal form Cu(I). As a consequence, yersiniabactin prevents catecholate’s toxic Cu(II) reduction and concomitantly allows their efficient Fe(III) uptake, thus conferring E. coli an optimal virulence.
- The siderophore yersiniabactin binds copper to protect pathogens during infection,
K. S. Chaturvedi, C. S. Hung, J. R. Crowley, A. E. Stapleton, J. P. Henderson,
Nat. Chem. Biol. 2012.
DOI: 10.1038/nchembio.102