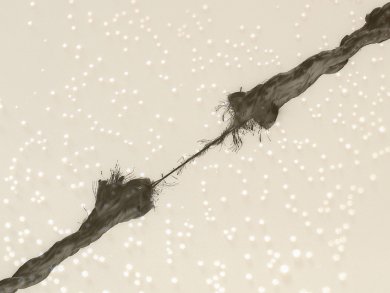
Ultrasound has been used widely in chemistry, but the mechanical aspects of its effects on polymers is only now being clarified. The advent of such mechanochemistry might open up new routes to novel compounds.
Chemists at the University of Oslo, Norway, were curious as to why their ultrasound reactions of polymeric triazoles revealed much shorter chains than they expected. They carried out a hybrid density functional theory assessment – in the common B3LYP implementation – of the ultrasound reaction and suggest that mechanochemistry is only partly to blame. The team says this DFT method provides accurate estimates of bonds up to the point where they break. The physical action of the ultrasound energy on the compounds does lead to stretching of the polymer chains and ultimately ring opening and snapping of those chains, a process that does not occur with heating in the absence of ultrasound.
The team suggests that solvent and/or radical effects must also contribute to the ring-opening processes.
- Ring opening vs direct bond scission of the chain in polymeric triazoles under the influence of an external force,
Hans Sverre Smalø, Einar Uggerud,
Chem. Commun. 2012.
DOI: 10.1039/C2CC34056A