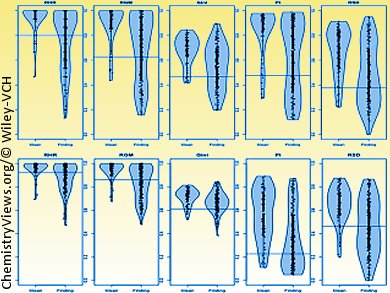
Computational methods for toxicity assessment are urgently sought to improve drug safety. The development of new drugs with fewer side-effects and greater efficacy increasingly relies on computational alternatives to in vivo animal studies. Using in vitro biochemical and cell-based assays, a compound’s activity spectrum can be rapidly measured. These data provide the basis for the development of predictive computer models of toxic effects.
Xiangyun Wang and Nigel Greene, Compound Safety Prediction Group at Pfizer Inc., USA, have found an elegant way to limit the number of biochemical experiments needed to predict the toxicity of a drug candidate by straightforward computational methods.
They compared various metrics of compound “promiscuity” and their relations to actual in vivo toxicity and found out that a small panel of only 15–30 tests is perfectly suited to achieve high prediction power. This new computational approach will have an impact in directing in vitro safety pharmacology testing to the optimum number of targets and thus help in the optimization process to deliver safer more efficient drug candidates.
- Comparing Measures of Promiscuity and Exploring Their Relationship to Toxicity,

Xiangyun Wang and Nigel Greene,
Mol. Inf. 2012, 31(2), 145–159.
DOI: 10.1002/minf.201100148
The paper won the Molecular Informatics Best Paper Award 2012.