Magnetite particles that arrange themselves into fine needles serve as a compass for some marine bacteria to orient themselves along the magnetic field of the Earth. Synthetic magnetite particles are also used in inks, magnetic liquids, medical contrast agent, and as memory elements in data storage media. Understanding how they grow could be helpful in generating nanoparticles with the desired properties.
In a supersaturated solution, several atoms and molecules agglomerate spontaneously into a seed that then grows further. Classical understanding says that the seed captures atoms or molecules from solution to either directly form a perfectly ordered crystal or an amorphous conglomeration which then rearranges itself into a crystal.
There have been increasing indications that numerous minerals like calcium carbonate and calcium phosphate that harden bones or mollusc shells do not grow according to this model. Instead they capture primary particles or cluster up to a few nanometres in size that only form temporarily.
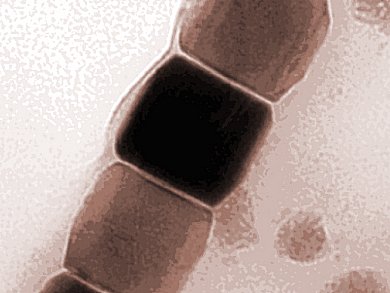
A team headed by Damien Faivre, Max Planck Institute of Colloids and Interfaces, Potsdam-Golm, Germany, studied magnetite nanoparticles with a transmission electron microscope operated at a temperature well below zero thus depicting especially small structures.
They developed a model of how crystalline particles form depending on their physical properties. Magnetite nanoparticles grow by absorbing small primary particles only 2 nm in size. The more stable the primary particles are, the more likely a crystalline structure forms directly. Whether crystals grow according to the classical model or the one proposed by Faivres’ team depends on whether atoms and molecules or the miniscule primary particles are involved.
Image: © MPI of Colloids and Interfaces
- Nucleation and growth of magnetite from solution,
Jens Baumgartner, Archan Dey, Paul H. H. Bomans, Cécile Le Coadou, Peter Fratzl, Nico A. J. M. Sommerdijk, Damien Faivre,
Nature Mat. 2013.
DOI: 10.1038/NMAT3558