Pericyclic cascade reactions are an efficient way to produce compact and complex structures with excellent stereocontrol. Consequently, such reactions are useful synthetic tools for chemists wanting to synthesize natural products with detailed molecular architectures.
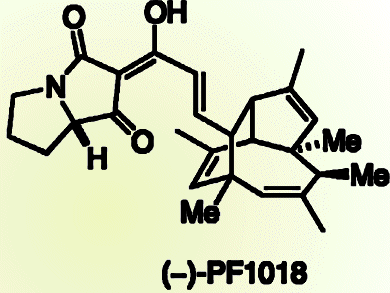
Dirk Trauner and colleagues from the Ludwig-Maximilians-Universität München and the Munich Center for Integrated Protein Science, Germany, synthesized the core of an insecticidal polyketide, (–)-PF-1018. The core is tricyclic and contains two quaternary and four secondary stereocenters, all of which are contiguous, and this complex target was formed by using a domino Stille/8π electrocyclization/Diels–Alder reaction sequence. A number of reaction outcomes were possible, but remarkably this asymmetric cascade led to a single diastereoisomer in synthetically useful yields (32 %).
Disappointingly, efforts so far to complete the synthesis of (–)-PF-1018, by dehydrating to introduce a double bond, have failed and alternative strategies are being sought.
- Studies toward the Biomimetic Total Synthesis of (−)-PF-1018,
Robert Webster, Boris Gaspar, Peter Mayer, Dirk Trauner
Org. Lett. 2013.
DOI: 10.1021/ol400508s