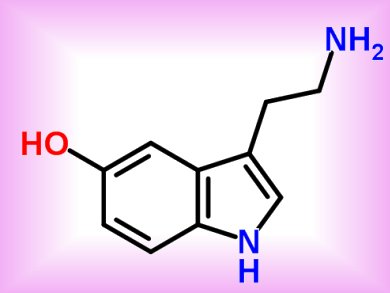
Serotonin receptors, transmembrane molecules activated by the neurotransmitter serotonin (5-hydroxytryptamine or 5-HT, pictured), are often pharmacologically stimulated to treat diseases such as depression. Nevertheless, if compounds targeting neuronal 5-HT receptors, such as 5-HT1B, aspecifically activate cardiovascular 5-HT2B receptors, they elicit harmful cardiotoxic side effects.
By examining 5-HT1B and 5-HT2B crystal structures, Chong Wang, Daniel Wacker, and colleagues, The Scripps Research Institute, La Jolla, USA, provided novel structural insights to design selective serotonergic drugs. The researchers compared the structure of the two molecules and demonstrated that they differ in an extracellular accessory binding pocket. In 5-HT2B receptors this pocket is narrower and slightly shifted. Therefore, it is less accessible to certain ligands such as the anti-migraine drug ergotamine. Thus, structural differences in this accessory binding pocket might be exploited to design drugs without cardiotoxic effects.
- Structural Basis for Molecular Recognition at Serotonin Receptors,
C. Wang, Y. Jiang, J. Ma, H. Wu, D. Wacker et al.,
Science 2013, 340 (6132), 610–614
DOI: 10.1126/science.1232807 - Structural Features for Functional Selectivity at Serotonin Receptors,
D. Wacker, C. Wang, V. Katritch, G. W. Han et al.,
Science 2013, 340 (6132), 615–619.
DOI: 10.1126/science.1232808