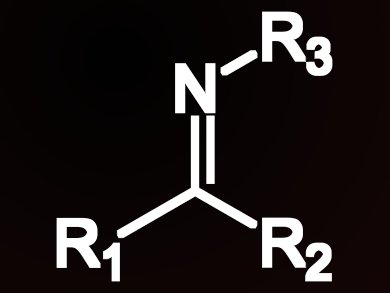
Amines are valuable compounds in both academic and industrial chemical synthesis, and are present in a vast number of bioactive compounds. Consequently, routes to synthesize amines attract attention, but relatively few routes starting from the corresponding imine exist.
Jianliang Xiao and colleagues, University of Liverpool, UK, have developed an iridium-containing metallocycle complex as a catalyst to reduce imines to amines under hydrogenation conditions. The complex, which contains an imino ligand, is highly active and selective for the hydrogenation of imines. A wide range of imine derivatives can be reduced quickly, typically < 1 h, and efficiently with 0.05 mol% catalyst loading under hydrogen gas (20 bar). The modular nature of the ligand means that the steric and electronic properties of the catalyst can be readily fine-tuned, with electron-withdrawing substituents enhancing activity.
The iridium complexes are easy to synthesize and air stable, and only simple work-up procedures are needed. These advantages, along with the use of hydrogen gas as a clean and scalable hydrogen source, make these catalysts attractive for academic and industrial applications alike.
- A Highly Active Cyclometallated Iridium Catalyst for the Hydrogenation of Imines,
Barbara Villa-Marcos, Weijun Tang, Xiaofeng Wu, Jianling Xiao,
Org. Biomolec. Chem. 2013.
DOI: 10.1039/C3OB41150H